Chapter 8 Concepts of Chemical Bonding
... electronegativity. The more polar bond will be the one between the atoms with the biggest differences in electronegativity. Consequently, the B—Cl bond is more polar; the chlorine atom carries the partial negative charge because it has a higher electronegativity. (b) In this example phosphorus is co ...
... electronegativity. The more polar bond will be the one between the atoms with the biggest differences in electronegativity. Consequently, the B—Cl bond is more polar; the chlorine atom carries the partial negative charge because it has a higher electronegativity. (b) In this example phosphorus is co ...
Optical modeling of finite element surface
... deviations to an optical surface. The data is assumed normal to the optical surface requiring finite element displacements to be converted into surface normal displacements. Interferogram file data may be represented in two formats - Zernike polynomials (Standard or Fringe) or as a uniform rectangul ...
... deviations to an optical surface. The data is assumed normal to the optical surface requiring finite element displacements to be converted into surface normal displacements. Interferogram file data may be represented in two formats - Zernike polynomials (Standard or Fringe) or as a uniform rectangul ...
DIFFUSION IN SOLIDS
... paths for atoms and ions. The concentration of these holes would have to be very great in order to account for the volume increase upon melting, thus resulting in much higher diffusion rates in liquids than in solids just below the melting point. Eyring theory: If the liquid is considered quasi-crys ...
... paths for atoms and ions. The concentration of these holes would have to be very great in order to account for the volume increase upon melting, thus resulting in much higher diffusion rates in liquids than in solids just below the melting point. Eyring theory: If the liquid is considered quasi-crys ...
ionization 12.3.1
... and electronic states and the electron has zero potential and kinetic energy. Electron energy The potential difference through which electrons are accelerated before they are used to bring about electron ionization. Fast atom bombardment ionization This term refers to the ionization of any species b ...
... and electronic states and the electron has zero potential and kinetic energy. Electron energy The potential difference through which electrons are accelerated before they are used to bring about electron ionization. Fast atom bombardment ionization This term refers to the ionization of any species b ...
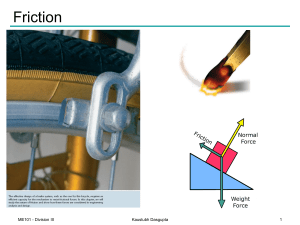
Adhesion

Adhesion is the tendency of dissimilar particles or surfaces to cling to one another (cohesion refers to the tendency of similar or identical particles/surfaces to cling to one another). The forces that cause adhesion and cohesion can be divided into several types. The intermolecular forces responsible for the function of various kinds of stickers and sticky tape fall into the categories of chemical adhesion, dispersive adhesion, and diffusive adhesion. In addition to the cumulative magnitudes of these intermolecular forces, there are certain emergent mechanical effects that will also be discussed at the end of the article.