File - Mr. Holz`s Website
... Ionic Bond – Transfer of electrons to create a bond between two ions that are attracted by opposite charges Covalent Bond – Bond that forms when electrons are shared between atoms Ion – Charged atoms that form from ionic bonds; atoms in which the number of electrons does not equal the number of prot ...
... Ionic Bond – Transfer of electrons to create a bond between two ions that are attracted by opposite charges Covalent Bond – Bond that forms when electrons are shared between atoms Ion – Charged atoms that form from ionic bonds; atoms in which the number of electrons does not equal the number of prot ...
Fall Final Review Honors
... point to identify the areas on which you need to spend more study time. For those areas, go back to homework assignments, quizzes, and reviews to practice more problems. I would also recommend going through all of your tests since these questions are only samples and do not include specific examples ...
... point to identify the areas on which you need to spend more study time. For those areas, go back to homework assignments, quizzes, and reviews to practice more problems. I would also recommend going through all of your tests since these questions are only samples and do not include specific examples ...
Unit 2: Biochem Notes
... sharp, sudden changes in pH. Buffers make acidic solutions more basic and basic solutions more acidic. Reactions occur best at a specific pH. - Humans must maintain a blood pH of 7.4 (+ or - .5). If the pH changes more than .5, then many chemical reactions in our cells would be negatively affected. ...
... sharp, sudden changes in pH. Buffers make acidic solutions more basic and basic solutions more acidic. Reactions occur best at a specific pH. - Humans must maintain a blood pH of 7.4 (+ or - .5). If the pH changes more than .5, then many chemical reactions in our cells would be negatively affected. ...
Unit 3C Standards for Quiz
... It will be similar to the last exam but there will be at least three questions per standard. Remember that since no calculators are allowed on the standards exam that we will be modeling this in this assessment of progress. Atomic and Molecular Structure 1. The Periodic Table displays the elements i ...
... It will be similar to the last exam but there will be at least three questions per standard. Remember that since no calculators are allowed on the standards exam that we will be modeling this in this assessment of progress. Atomic and Molecular Structure 1. The Periodic Table displays the elements i ...
research highlight in Nature Mat. (July 2011)
... viability could not yet be enhanced to unity, the findings may enable the development of more biocompatible quantum dots for imaging applications. ...
... viability could not yet be enhanced to unity, the findings may enable the development of more biocompatible quantum dots for imaging applications. ...
The Chemical Basis of Life
... – Other isotopes are radioactive, having unstable atoms that spontaneously break apart (decay) to form other atoms – When radioactive atoms decay, energy is released ...
... – Other isotopes are radioactive, having unstable atoms that spontaneously break apart (decay) to form other atoms – When radioactive atoms decay, energy is released ...
Standards Practice
... atoms in molecules such as Hz , CH4, NH3, HzCCHz , Nz, Clz, and many large biological molecules are covalent. 5. Which do not form covalent bonds? A. diatomic molecules B. large biological molecules C. molecules containing carbon D. salts 6. The bonds found in C2H4 are A. covalent. B. ionic. C. meta ...
... atoms in molecules such as Hz , CH4, NH3, HzCCHz , Nz, Clz, and many large biological molecules are covalent. 5. Which do not form covalent bonds? A. diatomic molecules B. large biological molecules C. molecules containing carbon D. salts 6. The bonds found in C2H4 are A. covalent. B. ionic. C. meta ...
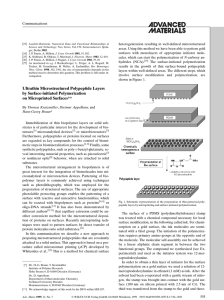
amcommu 555..558 - Leibniz-Institut für Polymerforschung Dresden
... remarkable that the geometrical features introduced by microprinting are retained accurately during the polymerization process. We prepared micropatterned peptide layers with individual repeating motifs with dimensions between 3.8 mm and 100 mm. From the structured surface it is possible to determin ...
... remarkable that the geometrical features introduced by microprinting are retained accurately during the polymerization process. We prepared micropatterned peptide layers with individual repeating motifs with dimensions between 3.8 mm and 100 mm. From the structured surface it is possible to determin ...
Adhesion

Adhesion is the tendency of dissimilar particles or surfaces to cling to one another (cohesion refers to the tendency of similar or identical particles/surfaces to cling to one another). The forces that cause adhesion and cohesion can be divided into several types. The intermolecular forces responsible for the function of various kinds of stickers and sticky tape fall into the categories of chemical adhesion, dispersive adhesion, and diffusive adhesion. In addition to the cumulative magnitudes of these intermolecular forces, there are certain emergent mechanical effects that will also be discussed at the end of the article.