Using your periodic table ppt (9/26-10/11) File
... • The electrons in the outer most shell of any element are called valance electrons. ...
... • The electrons in the outer most shell of any element are called valance electrons. ...
Masterton and Hurley Chapter 2
... • Weighted average is the addition of the contributions from each isotope • percent abundance is the percent or fraction of each isotope found in nature. ...
... • Weighted average is the addition of the contributions from each isotope • percent abundance is the percent or fraction of each isotope found in nature. ...
Bean Bag Lab
... Introduction: John Dalton’s atomic theory that stated all atoms of the same element are identical and equal in mass was simple yet revolutionary. Unfortunately, it was not quite right. More research started to show that atoms of the same element could have different masses. These atoms were call iso ...
... Introduction: John Dalton’s atomic theory that stated all atoms of the same element are identical and equal in mass was simple yet revolutionary. Unfortunately, it was not quite right. More research started to show that atoms of the same element could have different masses. These atoms were call iso ...
Grade 9 Science Unit: Atoms and Elements Topic 4: Periodic Table
... - Typically found ___________ the elements name in each element square - Atomic mass is always expressed as a _____________, because each element, except for _____________, can form _____________, which are atoms with different numbers of ____________ in them and therefore different ______________. ...
... - Typically found ___________ the elements name in each element square - Atomic mass is always expressed as a _____________, because each element, except for _____________, can form _____________, which are atoms with different numbers of ____________ in them and therefore different ______________. ...
Atoms and Molecules - Library Video Company
... everything look alike? The answer to this question can be found by studying the properties of elements. Atoms with identical properties and a specific number of protons in their nuclei are called elements. For example, every atom of the element sulfur in the universe has 16 protons and possesses ide ...
... everything look alike? The answer to this question can be found by studying the properties of elements. Atoms with identical properties and a specific number of protons in their nuclei are called elements. For example, every atom of the element sulfur in the universe has 16 protons and possesses ide ...
ELEMENTS AND SYMBOLS
... The number of protons in an atom determines its identity, and is called atomic number (Z). In a neutral atom, the number of protons (+) are equal to the number of electrons (–). Almost all the mass of the atom rests in the nucleus. Therefore the number of protons and neutrons in an atom is cal ...
... The number of protons in an atom determines its identity, and is called atomic number (Z). In a neutral atom, the number of protons (+) are equal to the number of electrons (–). Almost all the mass of the atom rests in the nucleus. Therefore the number of protons and neutrons in an atom is cal ...
Ch 2 Notes
... that atoms of different elements are different 3. Cmpds are formed when atoms of diff. elements combine, a given cmpd has same # & types of atoms 4. Rxns involve reorganization of atoms ...
... that atoms of different elements are different 3. Cmpds are formed when atoms of diff. elements combine, a given cmpd has same # & types of atoms 4. Rxns involve reorganization of atoms ...
History_of_the_Atomic_Model
... Atomic Models: Dalton 1. Elements composed of atoms; atoms are indestructible 2. Atoms of the same element are exactly alike 3. Atoms of different elements are different 4. Compounds formed by joining 2 atoms ...
... Atomic Models: Dalton 1. Elements composed of atoms; atoms are indestructible 2. Atoms of the same element are exactly alike 3. Atoms of different elements are different 4. Compounds formed by joining 2 atoms ...
5Periodic Table of Elements WB
... When a person is confronted with a large number of items, it is only natural to look for similarities that can be used to develop a classification scheme. A person who collects baseball cards may group his cards according to team or position. Biologists classify all living organisms in a fivekingdom ...
... When a person is confronted with a large number of items, it is only natural to look for similarities that can be used to develop a classification scheme. A person who collects baseball cards may group his cards according to team or position. Biologists classify all living organisms in a fivekingdom ...
4-1 Introduction to Atoms Directed Reading Questions
... 10. How are electrons in Bohr’s model like planets in the solar system? They move around the nucleus in specific orbits (p. 127) 11. Why was the idea of an “electron cloud” created? A visual model showing where electrons are located (p. 127) 12. What is a neutron? A subatomic particle with no charge ...
... 10. How are electrons in Bohr’s model like planets in the solar system? They move around the nucleus in specific orbits (p. 127) 11. Why was the idea of an “electron cloud” created? A visual model showing where electrons are located (p. 127) 12. What is a neutron? A subatomic particle with no charge ...
1 - Groupfusion.net
... 10. Explain the difference between a homogeneous mixture (solution) and a heterogeneous mixture. Give an example of each. Heterogeneous Mixture : A mixture that does not blend smoothly throughout – ex. sand and water Homogeneous Mixture: A mixture that has constant composition throughout – ex. salt ...
... 10. Explain the difference between a homogeneous mixture (solution) and a heterogeneous mixture. Give an example of each. Heterogeneous Mixture : A mixture that does not blend smoothly throughout – ex. sand and water Homogeneous Mixture: A mixture that has constant composition throughout – ex. salt ...
atomic mass
... • ORBITAL: the regions in an atom where there is a high probability of finding electrons. • s is the lowest energy orbital, and p is slightly higher ...
... • ORBITAL: the regions in an atom where there is a high probability of finding electrons. • s is the lowest energy orbital, and p is slightly higher ...
File - Science by Shaw
... The Atomic Mass Unit (amu) was created to measure the mass of p+, n0, and e-. ◦ 1 amu = 1/12 the mass of a carbon-12 atom ...
... The Atomic Mass Unit (amu) was created to measure the mass of p+, n0, and e-. ◦ 1 amu = 1/12 the mass of a carbon-12 atom ...
Ions - amyschaefer24
... element but have different masses. •Atoms that have the same number of protons but a different number of neutrons. •Why doesn’t an isotope form if we change the number of protons? ...
... element but have different masses. •Atoms that have the same number of protons but a different number of neutrons. •Why doesn’t an isotope form if we change the number of protons? ...
Atomic structure and periodic table review questions What is an
... 11.An element that has either a positive or negative charge is ___? 12.What is a positively charged element called? 13.An anion is a ____. 14.If an element has a negative charge, it has more ____ than ____. 15.Atoms of the same element that have different numbers of neutrons are called ___. 16.What ...
... 11.An element that has either a positive or negative charge is ___? 12.What is a positively charged element called? 13.An anion is a ____. 14.If an element has a negative charge, it has more ____ than ____. 15.Atoms of the same element that have different numbers of neutrons are called ___. 16.What ...
Reporting Category 2: Atomic Structure and Nuclear Chemistry
... • Elements are made of very small, indivisible particles called atoms. • All atoms of a given element are identical. • Atoms of a given element are different from those of any other element and have different atomic masses. • In a chemical reaction, atoms of one element can combine with atoms of ano ...
... • Elements are made of very small, indivisible particles called atoms. • All atoms of a given element are identical. • Atoms of a given element are different from those of any other element and have different atomic masses. • In a chemical reaction, atoms of one element can combine with atoms of ano ...
K,7th Grade Test Review: Atoms and Chemical Reactions PART
... PART FOUR: Chemical Equations. For each equation, label the products and reactants. Then, count the number of atoms of each element on each side. Then fill in the blanks. ...
... PART FOUR: Chemical Equations. For each equation, label the products and reactants. Then, count the number of atoms of each element on each side. Then fill in the blanks. ...
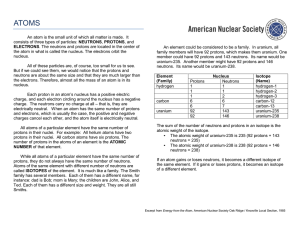
An atom is the small unit of which all matter is made. It consists of
... the atom in what is called the nucleus. The electrons orbit the nucleus. All of these particles are, of course, too small for us to see. But if we could see them, we would notice that the protons and neutrons are about the same size and that they are much larger than the electrons. Therefore, almost ...
... the atom in what is called the nucleus. The electrons orbit the nucleus. All of these particles are, of course, too small for us to see. But if we could see them, we would notice that the protons and neutrons are about the same size and that they are much larger than the electrons. Therefore, almost ...