Study on off-label use of medicinal products in the European Union
... the off-label use of medicinal products in various EU Member States. This includes describing how authorities have addressed the issue and the different ways patients, healthcare professionals and industry have reacted to this; 3. Providing a factual analysis taking into account the EU legal framewo ...
... the off-label use of medicinal products in various EU Member States. This includes describing how authorities have addressed the issue and the different ways patients, healthcare professionals and industry have reacted to this; 3. Providing a factual analysis taking into account the EU legal framewo ...
Study on off-label use of medicinal products in the European
... the off-label use of medicinal products in various EU Member States. This includes describing how authorities have addressed the issue and the different ways patients, healthcare professionals and industry have reacted to this; 3. Providing a factual analysis taking into account the EU legal framewo ...
... the off-label use of medicinal products in various EU Member States. This includes describing how authorities have addressed the issue and the different ways patients, healthcare professionals and industry have reacted to this; 3. Providing a factual analysis taking into account the EU legal framewo ...
The use of natural and synthetic phospholipids as pharmaceutical
... chiefly of PC, PE, phosphatidylserine, and phosphatidylinositol, combined with various amounts of other substances such as triglycerides, fatty acids, and carbohydrates, as separated from the crude vegetable oil source. It contains not less than 50% of acetone‐insoluble matter.” Normally, lecithin gr ...
... chiefly of PC, PE, phosphatidylserine, and phosphatidylinositol, combined with various amounts of other substances such as triglycerides, fatty acids, and carbohydrates, as separated from the crude vegetable oil source. It contains not less than 50% of acetone‐insoluble matter.” Normally, lecithin gr ...
Ciprofloxacin hydrochloride
... therapeutic use and index, pharmacokinetics, excipient interactions and reported BE/bioavailability (BA) problems were taken into consideration. Solubility and BA data indicate that ciprofloxacin hydrochloride is a BCS Class IV drug. Therefore, a biowaiver based approval of ciprofloxacin hydrochlori ...
... therapeutic use and index, pharmacokinetics, excipient interactions and reported BE/bioavailability (BA) problems were taken into consideration. Solubility and BA data indicate that ciprofloxacin hydrochloride is a BCS Class IV drug. Therefore, a biowaiver based approval of ciprofloxacin hydrochlori ...
Prescription medicine use by one million Canadian children
... as a problem for many years. This has been a significant burden for child health care providers and has meant that therapy for children has, on average, lagged behind that available for routine use by adults. The reasons for the dearth of drug information for children are many and include lack of re ...
... as a problem for many years. This has been a significant burden for child health care providers and has meant that therapy for children has, on average, lagged behind that available for routine use by adults. The reasons for the dearth of drug information for children are many and include lack of re ...
Esam Z. Dajani, Ph.D., FACG
... worldwide clinical research and development (Phases I-IV). Provided consultation services and actively interacted with clinical, regulatory affairs, legal, marketing, business development and licensing groups. Director, Cytotec Scientific and Medical Affairs, G.D. Searle and Co., Skokie, Illinois. 1 ...
... worldwide clinical research and development (Phases I-IV). Provided consultation services and actively interacted with clinical, regulatory affairs, legal, marketing, business development and licensing groups. Director, Cytotec Scientific and Medical Affairs, G.D. Searle and Co., Skokie, Illinois. 1 ...
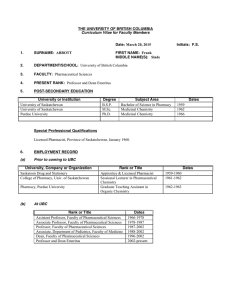
CV - Faculty of Pharmaceutical Sciences
... and more often very sensitive methods of analysis. This is particularly true for the anticonvulsant valproic acid. An assay has been developed for seventeen of these metabolites using SIM techniques and stable isotope analogs as internal standards. The assay is now being applied to patient studies, ...
... and more often very sensitive methods of analysis. This is particularly true for the anticonvulsant valproic acid. An assay has been developed for seventeen of these metabolites using SIM techniques and stable isotope analogs as internal standards. The assay is now being applied to patient studies, ...
Pfizer Guitly of Violating California Unfair Competition Law
... FDA on how to conduct clinical trials. He is currently a senior advisor to the dean at Wake Forest University School of Medicine, where he previously established a research center on epidemiology and biostatistics. Dr. Furberg is the co-author of Fundamentals of Clinical Trials, the leading text in ...
... FDA on how to conduct clinical trials. He is currently a senior advisor to the dean at Wake Forest University School of Medicine, where he previously established a research center on epidemiology and biostatistics. Dr. Furberg is the co-author of Fundamentals of Clinical Trials, the leading text in ...
capecitabine - Medicines and Healthcare products Regulatory Agency
... Film-coated Tablets. Two medicines are bioequivalent when they produce the same levels of the active substance in the body. What are the benefits and risks of Capecitabine Reliance Tablets? Because Capecitabine Reliance Tablets are generic medicines and are bioequivalent to the reference medicines, ...
... Film-coated Tablets. Two medicines are bioequivalent when they produce the same levels of the active substance in the body. What are the benefits and risks of Capecitabine Reliance Tablets? Because Capecitabine Reliance Tablets are generic medicines and are bioequivalent to the reference medicines, ...
P M harmacist’s anual
... This manual has been prepared by the Drug Enforcement Administration, Office of Diversion Control, as a guide to assist pharmacists in their understanding of the Federal Controlled Substances Act and its implementing regulations as they pertain to the pharmacy profession. The 2010 edition replaces a ...
... This manual has been prepared by the Drug Enforcement Administration, Office of Diversion Control, as a guide to assist pharmacists in their understanding of the Federal Controlled Substances Act and its implementing regulations as they pertain to the pharmacy profession. The 2010 edition replaces a ...
IN THE UNITED STATES DISTRICT COURT WESTERN DISTRICT
... that the cardiovascular events “are clearly there” and called it a “shame.” Wall Street Journal, Nov. 1, 2004, p. A1. Merck’s public statements (and specifically to prescribing doctors, through literature and sales rep visits, such as Mr. Hodge’s prescribing physician, M.D.) continued to reject any ...
... that the cardiovascular events “are clearly there” and called it a “shame.” Wall Street Journal, Nov. 1, 2004, p. A1. Merck’s public statements (and specifically to prescribing doctors, through literature and sales rep visits, such as Mr. Hodge’s prescribing physician, M.D.) continued to reject any ...
The Business Benefits of Quality by Design (QbD) Business Benefits of QbD
... regulators would be inspecting the manufacturing site and so the company wanted to achieve a high level of confidence from the regulators regarding the site’s approach and capability. • It is company policy now, but the history is that the approach was driven by a relative small group of like-minde ...
... regulators would be inspecting the manufacturing site and so the company wanted to achieve a high level of confidence from the regulators regarding the site’s approach and capability. • It is company policy now, but the history is that the approach was driven by a relative small group of like-minde ...
DXM
... Despite growing awareness of the prevalence of DXM use, little systematic information is available on use patterns and preferences among recreational DXM users, on such users’ perceptions and attitudes toward DXM use, or on the impact of the Internet on DXM use. Given DXM’s legal availability, bette ...
... Despite growing awareness of the prevalence of DXM use, little systematic information is available on use patterns and preferences among recreational DXM users, on such users’ perceptions and attitudes toward DXM use, or on the impact of the Internet on DXM use. Given DXM’s legal availability, bette ...
P M harmacist’s anual
... Appendix C Definitions of Abbreviations ....................................................................................... 65 Appendix D Pharmacist’s Guide to Prescription Fraud ................................................................. 66 Appendix E Affidavit for a New Pharmacy ........ ...
... Appendix C Definitions of Abbreviations ....................................................................................... 65 Appendix D Pharmacist’s Guide to Prescription Fraud ................................................................. 66 Appendix E Affidavit for a New Pharmacy ........ ...
Improving anti-drug antibody assay performance in
... Today mAbs are used as targeting therapies for several diseases including autoimmune and infectious diseases, oncological disorders and transplant rejection (Hansel et al. 2010). In 2012 Sloan et al. reported that over 20 mAbs have been approved and that more than 200 are in development. One advanta ...
... Today mAbs are used as targeting therapies for several diseases including autoimmune and infectious diseases, oncological disorders and transplant rejection (Hansel et al. 2010). In 2012 Sloan et al. reported that over 20 mAbs have been approved and that more than 200 are in development. One advanta ...
July 28, 2014 Margaret A. Hamburg, MD
... GRASE when indicated, among other things, “[f]or the temporary relief of sore gums due to teething in infants and children 4 months of age and older.” 36 In making its GRASE determination, the Panel recognized that benzocaine is widely used as a topical anesthetic but did not discuss any studies of ...
... GRASE when indicated, among other things, “[f]or the temporary relief of sore gums due to teething in infants and children 4 months of age and older.” 36 In making its GRASE determination, the Panel recognized that benzocaine is widely used as a topical anesthetic but did not discuss any studies of ...
COMPARATIVE EVALUATION OF NATURAL AND SYNTHETIC SUPERDISINTEGRANT FOR PROMOTING NIMESULIDE DISSOLUTION FOR FAST DISSOLVING TECHNOLOGY
... Now a days fast dissolving technology has a nice applicability in case of patient care. Because this type of formulation can disintegrate within few seconds and release their active ingredient very fast and onset of action can be achieved in few minutes. Mostly superdisintegran ...
... Now a days fast dissolving technology has a nice applicability in case of patient care. Because this type of formulation can disintegrate within few seconds and release their active ingredient very fast and onset of action can be achieved in few minutes. Mostly superdisintegran ...
JIPBS Simple UV spectrophotometric assay of Furosemide Original Article
... failure, when we require rapid diuretic action and intestinal absorption may be delayed because of gastrointestinal oedema Thus, time to peak, lag time and peak of serum concentration may differ in compensated when compared with decompensate patients after the oral furosemide is intake, whereas elim ...
... failure, when we require rapid diuretic action and intestinal absorption may be delayed because of gastrointestinal oedema Thus, time to peak, lag time and peak of serum concentration may differ in compensated when compared with decompensate patients after the oral furosemide is intake, whereas elim ...