9081872 Physics Jan. 01
... 4 Approximately how far will an object near Earth’s surface fall in 3.0 seconds? (1) 88 m (3) 29 m (2) 44 m (4) 9.8 m ...
... 4 Approximately how far will an object near Earth’s surface fall in 3.0 seconds? (1) 88 m (3) 29 m (2) 44 m (4) 9.8 m ...
AP Physics 2: Algebra-Based 2015 Free
... ii. Before the switch is closed, the power expended by bulb 1 is P1 . Derive an expression for the power Pnew expended by bulb 1 after the switch is closed in terms of P1 . iii. How does the result of your derivation in part (a)ii relate to your explanation in part (a)i? (b) A student makes the foll ...
... ii. Before the switch is closed, the power expended by bulb 1 is P1 . Derive an expression for the power Pnew expended by bulb 1 after the switch is closed in terms of P1 . iii. How does the result of your derivation in part (a)ii relate to your explanation in part (a)i? (b) A student makes the foll ...
Static
... Induction: Consider two insulated metal spheres A and B. a. They touch each other, so in effect they form a single uncharged conductor. b. When a negatively charged rod is brought near A, electrons in the metal, being free to move, are repelled as far as possible until their mutual repulsion is big ...
... Induction: Consider two insulated metal spheres A and B. a. They touch each other, so in effect they form a single uncharged conductor. b. When a negatively charged rod is brought near A, electrons in the metal, being free to move, are repelled as far as possible until their mutual repulsion is big ...
Chapter 3 - WebAssign
... while the valence orbitals are used to accept electrons from or to share electrons with other atoms. Thus, the number and location of the valence electrons are important characteristics of the atom, and they are given in an atom’s valence electron configuration. As shown in Table 3.1, the number of ...
... while the valence orbitals are used to accept electrons from or to share electrons with other atoms. Thus, the number and location of the valence electrons are important characteristics of the atom, and they are given in an atom’s valence electron configuration. As shown in Table 3.1, the number of ...
Doped semiconductors: donor impurities
... As compared to Si, the Phosphorus has one extra valence electron which, after all bonds are made, has very weak bonding. Very small energy is required to create a free electron from an impurity atom. This type of impurity is called donor. Note, that there is no hole created when a free electron come ...
... As compared to Si, the Phosphorus has one extra valence electron which, after all bonds are made, has very weak bonding. Very small energy is required to create a free electron from an impurity atom. This type of impurity is called donor. Note, that there is no hole created when a free electron come ...
Self Force on Accelerated Charged Particle
... derived without Lienard-Wiechert fields as done for example by Purcell [14]. Unexpected solutions can be found to long standing problems [15]. The analysis in the present paper is well suited for the electron because it is structureless and point-like, but should be a good approximation for structur ...
... derived without Lienard-Wiechert fields as done for example by Purcell [14]. Unexpected solutions can be found to long standing problems [15]. The analysis in the present paper is well suited for the electron because it is structureless and point-like, but should be a good approximation for structur ...
and n
... NB: Each carbon contributes one electron to the π system while the two N atoms contribute 3 electrons. ...
... NB: Each carbon contributes one electron to the π system while the two N atoms contribute 3 electrons. ...
Part VII
... the Pauli exclusion principle postulates that only one electron can occupy a single state therefore, as electrons are added to a system, they will fill the states in a system like water fills a bucket – first the lower energy states and then the higher energy states the ground state of the N-electro ...
... the Pauli exclusion principle postulates that only one electron can occupy a single state therefore, as electrons are added to a system, they will fill the states in a system like water fills a bucket – first the lower energy states and then the higher energy states the ground state of the N-electro ...
Fine and hyperfine structure of the hydrogen atom
... Hence, for the doubly degenerate eigenstate of the Hamiltonian H0 + Wf s the inclusion of Wz serves to lift the degeneracy, separating these states by ~ωL . Having sufficiently warmed up now, let us move on now to the more complicated 2p state. As one may recall, the degeneracy of the six 2p states ...
... Hence, for the doubly degenerate eigenstate of the Hamiltonian H0 + Wf s the inclusion of Wz serves to lift the degeneracy, separating these states by ~ωL . Having sufficiently warmed up now, let us move on now to the more complicated 2p state. As one may recall, the degeneracy of the six 2p states ...
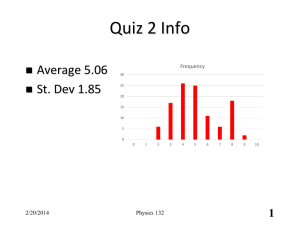
Physics 132 Prof. Buehrle 2/11/14
... S1 and S2 decrease by equal amounts. S1 and S2 decrease by unequal amounts. S1 increases, but S2 decreases by more. S1 increases and S2 decreases by equal amounts. None of the above exchanges occur. ...
... S1 and S2 decrease by equal amounts. S1 and S2 decrease by unequal amounts. S1 increases, but S2 decreases by more. S1 increases and S2 decreases by equal amounts. None of the above exchanges occur. ...
AS Physics Paper March 2016
... Interplanetary satellites are very complex platforms with dozens of scientific instruments, mechanical devices and radio transmitters and receivers on board. They require considerable power and operate over many years, and those travelling to the outer planets cannot use solar power. They rely inste ...
... Interplanetary satellites are very complex platforms with dozens of scientific instruments, mechanical devices and radio transmitters and receivers on board. They require considerable power and operate over many years, and those travelling to the outer planets cannot use solar power. They rely inste ...
Motion in a Straight Line
... The screen of old fashioned TVs is coated on the inside surface with dots of chemicals called phosphors. When a beam of electrons hits a dot, it glows. These phosphor dots are in groups of three: Red, Green, and Blue which then create all the other colours by combining which dots are illuminated. Th ...
... The screen of old fashioned TVs is coated on the inside surface with dots of chemicals called phosphors. When a beam of electrons hits a dot, it glows. These phosphor dots are in groups of three: Red, Green, and Blue which then create all the other colours by combining which dots are illuminated. Th ...
Worked solutions
... 13. Now both slits are unblocked. However, we modify the experiment in the following way: We prepare the electrons incident on the slits so that they all have their spins “pointing up”, i.e., so that ms = +1/2. We install a tiny radio-coil near the top slit (this is only a thought experiment!), so ...
... 13. Now both slits are unblocked. However, we modify the experiment in the following way: We prepare the electrons incident on the slits so that they all have their spins “pointing up”, i.e., so that ms = +1/2. We install a tiny radio-coil near the top slit (this is only a thought experiment!), so ...
Physics 30 - Paul Rowe JrSr High School
... explain, qualitatively, how electron diffraction provides experimental support for the de Broglie hypothesis describe, qualitatively, how the two-slit electron interference experiment shows that quantum systems, like photons and electrons, may be modelled as particles or waves, contrary to intui ...
... explain, qualitatively, how electron diffraction provides experimental support for the de Broglie hypothesis describe, qualitatively, how the two-slit electron interference experiment shows that quantum systems, like photons and electrons, may be modelled as particles or waves, contrary to intui ...
chemistry chapter
... exclusion principle which states that no two electrons in an atom can have the same four quantum numbers. Knowledge of the four quantum numbers will help you decide the electronic configuration (arrangement of electrons) in a given atom. The principal quantum number (n) indicates the main energy lev ...
... exclusion principle which states that no two electrons in an atom can have the same four quantum numbers. Knowledge of the four quantum numbers will help you decide the electronic configuration (arrangement of electrons) in a given atom. The principal quantum number (n) indicates the main energy lev ...
Physics 3 Revision GUide
... 2. The incident angle θ1 equal to the critical angle and so the light ray passes along the surface of the boundary. 3. The incident angle is greater than the critical angle and so the light ray is reflected back into the water. This phenomenon is known as total internal reflection. θ1 = θ2 ...
... 2. The incident angle θ1 equal to the critical angle and so the light ray passes along the surface of the boundary. 3. The incident angle is greater than the critical angle and so the light ray is reflected back into the water. This phenomenon is known as total internal reflection. θ1 = θ2 ...
gamma - radiation connected to atmospheric precipitations.
... In several cases of considerable X-ray increases the samples of precipitations in the form of rain and snow have been collected and analyzed with radiochemical methods in the regional laboratory of radiochemical control. This laboratory carries out regular measurements of all radionuclides, both nat ...
... In several cases of considerable X-ray increases the samples of precipitations in the form of rain and snow have been collected and analyzed with radiochemical methods in the regional laboratory of radiochemical control. This laboratory carries out regular measurements of all radionuclides, both nat ...
Quantum Mechanics Unit Review Answers AP Physics
... there was no need to adjust the model simply to include that revolutionary idea. However, the quantum mechanical model of the atom does make two major changes to Bohr’s model. First it does away with Bohr’s orbits because the Heisenberg uncertainty principle won’t allow enough precision in an electr ...
... there was no need to adjust the model simply to include that revolutionary idea. However, the quantum mechanical model of the atom does make two major changes to Bohr’s model. First it does away with Bohr’s orbits because the Heisenberg uncertainty principle won’t allow enough precision in an electr ...
Atomic Structure and Periodicity – web
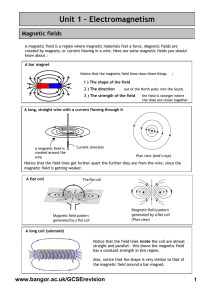
... • When all of the domains, represented by the arrows, are aligned, it behaves as a magnet. • If you drop it: The domains go in all different directions and it no longer operates as a magnet. ...
... • When all of the domains, represented by the arrows, are aligned, it behaves as a magnet. • If you drop it: The domains go in all different directions and it no longer operates as a magnet. ...