Advanced Higher - Hodder Education
... published SQA questions, has been specially commissioned by Hodder Gibson, and has been written by experienced senior teachers and examiners. This is not SQA material but has been devised to provide further practice for SQA National Qualification examinations in 2016 and beyond. Model Question Paper ...
... published SQA questions, has been specially commissioned by Hodder Gibson, and has been written by experienced senior teachers and examiners. This is not SQA material but has been devised to provide further practice for SQA National Qualification examinations in 2016 and beyond. Model Question Paper ...
National German competition
... Apart from water, other chemical solvents are frequently used as well. Liquid ammonia is one of them that has been investigated best. Like water molecules, ammonia molecules are polar as well. Thus, ammonia can dissolve ionic compounds well. There is an autoprtolysis equilibrium in liquid ammonia: 2 ...
... Apart from water, other chemical solvents are frequently used as well. Liquid ammonia is one of them that has been investigated best. Like water molecules, ammonia molecules are polar as well. Thus, ammonia can dissolve ionic compounds well. There is an autoprtolysis equilibrium in liquid ammonia: 2 ...
Supporting Information - Royal Society of Chemistry
... concentrations of inhibitors. For clarity, such plots are shown only for selected concentrations of inhibitors (Figure S3). The double reciprocal plots revealed that all inhibitors (complexes 2-4) were competitive types. However, since the concentration of the enzyme utilized during the above experi ...
... concentrations of inhibitors. For clarity, such plots are shown only for selected concentrations of inhibitors (Figure S3). The double reciprocal plots revealed that all inhibitors (complexes 2-4) were competitive types. However, since the concentration of the enzyme utilized during the above experi ...
Energy is the essence of chemistry It determines which reaction can
... • A negative ion is next to a positive ion. The strong attraction between these ions causes a high melting and boiling temperature. Many ionic solids are soluble in water An electrolyte solution. It conducts electricity Many are also sparingly soluble in water Calcium phosphate in bones is very spar ...
... • A negative ion is next to a positive ion. The strong attraction between these ions causes a high melting and boiling temperature. Many ionic solids are soluble in water An electrolyte solution. It conducts electricity Many are also sparingly soluble in water Calcium phosphate in bones is very spar ...
equilibrium - chemistryatdulwich
... How does Kc indicate position of equilibrium? We should really accept that each reaction, including those going to a virtual completion and those seemingly not starting at all, have a tendency to attain a position of equilibrium and have therefore a K value. If Kc 1 than [products] exceed [reacta ...
... How does Kc indicate position of equilibrium? We should really accept that each reaction, including those going to a virtual completion and those seemingly not starting at all, have a tendency to attain a position of equilibrium and have therefore a K value. If Kc 1 than [products] exceed [reacta ...
Solubility Solubility is defined as the amount of solute that will
... When a solid is allowed to come to equilibrium in a solution of one of it constituent ions we call this the common ion effect. As we would expect from LeChatlier’s principle addition of a product ( the common ion) would shift the equilibrim to the reactant side. An example would be letting solid sil ...
... When a solid is allowed to come to equilibrium in a solution of one of it constituent ions we call this the common ion effect. As we would expect from LeChatlier’s principle addition of a product ( the common ion) would shift the equilibrim to the reactant side. An example would be letting solid sil ...
20. Chemical Equilibrium
... a given temperature in such a way as to allow the products to accumulate in the reaction container. After a period of time, the reaction will reach equilibrium. At this point, it may be possible to experimentally determine the concentrations of the reactants and products in the container. The concen ...
... a given temperature in such a way as to allow the products to accumulate in the reaction container. After a period of time, the reaction will reach equilibrium. At this point, it may be possible to experimentally determine the concentrations of the reactants and products in the container. The concen ...
Chapter 5 Geochemical Weathering
... ferromagnesian silicates like basalts that dominate in volcanic belts. The kinetics of silicate weathering are slow, with the formation of secondary clays ― hydrated aluminosilcate minerals with high capacity for cation exchange and sorption. Weathering of carbonate rocks proceeds more rapidly than ...
... ferromagnesian silicates like basalts that dominate in volcanic belts. The kinetics of silicate weathering are slow, with the formation of secondary clays ― hydrated aluminosilcate minerals with high capacity for cation exchange and sorption. Weathering of carbonate rocks proceeds more rapidly than ...
alkalinity of groundwater samples
... may contribute to the alkalinity. Alkalinity can be measured as either the ‘phenolphthalein’ alkalinity (neutralization to a pH ~ 8.3) or as the ‘total’ alkalinity (pH ~ 4.2). The phenolphthalein alkalinity, written as [alk]P when expressed as the number of moles of H+ neutralized per litre, is equa ...
... may contribute to the alkalinity. Alkalinity can be measured as either the ‘phenolphthalein’ alkalinity (neutralization to a pH ~ 8.3) or as the ‘total’ alkalinity (pH ~ 4.2). The phenolphthalein alkalinity, written as [alk]P when expressed as the number of moles of H+ neutralized per litre, is equa ...
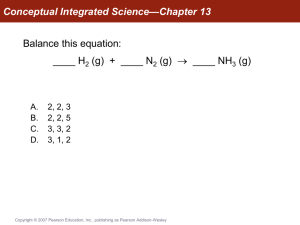
Conceptual Integrated Science—Chapter 13
... What is the relationship between a hydroxide ion and a water molecule? A. A hydroxide ion is a water molecule plus a proton. B. A hydroxide ion and a water molecule are the same things. C. A hydroxide ion is a water molecule minus a hydrogen nucleus. D. A hydroxide ion is a water molecule plus two e ...
... What is the relationship between a hydroxide ion and a water molecule? A. A hydroxide ion is a water molecule plus a proton. B. A hydroxide ion and a water molecule are the same things. C. A hydroxide ion is a water molecule minus a hydrogen nucleus. D. A hydroxide ion is a water molecule plus two e ...
Mole and Energy - Deans Community High School
... Thermochemistry is the study of heat energy taken in or given out in chemical reactions. This heat, absorbed or released, can be related to the internal energy of the substances involved. Such internal energy is called ENTHALPY, symbol H. As it is only possible to measure the change in enthalpy, the ...
... Thermochemistry is the study of heat energy taken in or given out in chemical reactions. This heat, absorbed or released, can be related to the internal energy of the substances involved. Such internal energy is called ENTHALPY, symbol H. As it is only possible to measure the change in enthalpy, the ...
Chemistry 12 Worksheet 2-3 Calculations Involving the
... given that the partial pressure of each substance at equilibrium is as follows: Partial Pressure of A2 = 20.0 kPa, Partial Pressure of B 2 = 30.0 kPa, Partial Pressure of ...
... given that the partial pressure of each substance at equilibrium is as follows: Partial Pressure of A2 = 20.0 kPa, Partial Pressure of B 2 = 30.0 kPa, Partial Pressure of ...
Soft X-Ray-Induced Decomposition of Amino Acids: An XPS, Mass
... spectra have complicated asymmetric shapes due to contributions of several functional groups and shake-up satellites.3 For the assignment of the spectral features, it must be taken into account that the form of amino acids that is most stable in the solid state is a zwitterion with a protonated amin ...
... spectra have complicated asymmetric shapes due to contributions of several functional groups and shake-up satellites.3 For the assignment of the spectral features, it must be taken into account that the form of amino acids that is most stable in the solid state is a zwitterion with a protonated amin ...
Chemical Reaction Equations
... Reactions are fast – the reaction must occur within a reasonable time (see pg. 280) Reactions are quantitative – one that is more than 99% complete; in other words, at least one reactant is completely used up Reactions are stoichiometric – means that there is a simple whole-number ratio of chemical ...
... Reactions are fast – the reaction must occur within a reasonable time (see pg. 280) Reactions are quantitative – one that is more than 99% complete; in other words, at least one reactant is completely used up Reactions are stoichiometric – means that there is a simple whole-number ratio of chemical ...
Study Guide for Module 11B—Solutions II
... ■Review of Preparing Solutions (From Module 4) Note: The procedure used to prepare the solution is the same as that taught in Chemistry 1010 and the accompanying laboratory. It is: Step 1: Measure out ___ g of the solute (or ___ ml of the original solution). Step 2: Put approximately one-half of the ...
... ■Review of Preparing Solutions (From Module 4) Note: The procedure used to prepare the solution is the same as that taught in Chemistry 1010 and the accompanying laboratory. It is: Step 1: Measure out ___ g of the solute (or ___ ml of the original solution). Step 2: Put approximately one-half of the ...