Organic Compounds - Ms. Nevel's Biology Website
... bones and muscles, Form parts of cell membranes, Function as hormones to regulate the body, Form antibodies to protect against infection, Increase the rate of chemical reactions to name a few. ...
... bones and muscles, Form parts of cell membranes, Function as hormones to regulate the body, Form antibodies to protect against infection, Increase the rate of chemical reactions to name a few. ...
Protein degradation in mouse brain slices
... a role for neurotoxic and unusual neuroexitatory amino acids in the aetiology of certain neurodegenerative disordcrs (Spencer er ul., 1987). This has led us to speculate whether those amino acids that are implicated as possible causativc o r contributory agents in these diseases, might also be invol ...
... a role for neurotoxic and unusual neuroexitatory amino acids in the aetiology of certain neurodegenerative disordcrs (Spencer er ul., 1987). This has led us to speculate whether those amino acids that are implicated as possible causativc o r contributory agents in these diseases, might also be invol ...
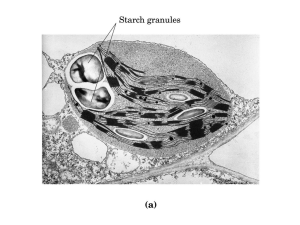
Cellular compartmentalization
... special sequences (signal sequences - 12 to 60 amino acids long) that are like barcodes. These are read by special courier protein systems within the cytoplasm and then targeted to their destinations. ...
... special sequences (signal sequences - 12 to 60 amino acids long) that are like barcodes. These are read by special courier protein systems within the cytoplasm and then targeted to their destinations. ...
Extraction and Purification
... – Exploits the different density of organelles – Density gradients are formed by using sucrose as solute – Can be step gradient or continuous – Centrifuge for set time at a know force and determine where your compound is or run it until it reaches equilibrium. Sample will stop moving once its densit ...
... – Exploits the different density of organelles – Density gradients are formed by using sucrose as solute – Can be step gradient or continuous – Centrifuge for set time at a know force and determine where your compound is or run it until it reaches equilibrium. Sample will stop moving once its densit ...
From DNA to Protein
... identity and position of each amino acid in the protein chain is coded by three consecutive nucleotides on the DNA, called codons. (DNA is made of four nucleotides, adenine, thymine, guanine and cytosine, abbreviated with letters A, T, G, C - the first letters of their names). Three nucleotides make ...
... identity and position of each amino acid in the protein chain is coded by three consecutive nucleotides on the DNA, called codons. (DNA is made of four nucleotides, adenine, thymine, guanine and cytosine, abbreviated with letters A, T, G, C - the first letters of their names). Three nucleotides make ...
Some funcaon of proteins
... The carbonyl oxygen has a par9al nega9ve charge and the amide nitrogen a par9al posi9ve change, se_ng up a small electric dipole. Virtually all pep9de bonds in proteins occur in this trans configura9on ...
... The carbonyl oxygen has a par9al nega9ve charge and the amide nitrogen a par9al posi9ve change, se_ng up a small electric dipole. Virtually all pep9de bonds in proteins occur in this trans configura9on ...
Key: Biomolecule Study Guide 1) In animals, excess carbohydrates
... They must fit the molecules that they interact with (Lock and Key) 10) What does it mean to say an enzyme is “denatured”? It has changed its 3-D shape 11) What are 2 ways to denature an enzyme? Change the temperature or pH ...
... They must fit the molecules that they interact with (Lock and Key) 10) What does it mean to say an enzyme is “denatured”? It has changed its 3-D shape 11) What are 2 ways to denature an enzyme? Change the temperature or pH ...
BCBT100 Biochemistry of Food Study Guide
... (http://www.queenoflub.com/biochem/terms.html). I think it will be very helpful if you can understand more than the vocabulary. The best way to prepare is to look at each bullet ...
... (http://www.queenoflub.com/biochem/terms.html). I think it will be very helpful if you can understand more than the vocabulary. The best way to prepare is to look at each bullet ...
What is the average % of protein in Grade 1 oats
... -Increases tastiness of food supplements -Increases stamina in performance horses ...
... -Increases tastiness of food supplements -Increases stamina in performance horses ...
What are you made of?
... blood causes a “need” for that molecule • Cross into cells through diffusion or through active transport ...
... blood causes a “need” for that molecule • Cross into cells through diffusion or through active transport ...
Amino Acids and Proteins
... Helps some proteins as water hating ‘hydrophobic’ groups point inwards, therefore hold structure together Occurs between non-polar side chains ...
... Helps some proteins as water hating ‘hydrophobic’ groups point inwards, therefore hold structure together Occurs between non-polar side chains ...
lecture-5-Proteins and their structure
... bend in proline allow the polypeptide backbone to fold into a tight U shape. Turns allow large proteins to fold into highly compact structures. A polypeptide backbone also may contain longer bends, or loops. In contrast with turns, which exhibit just a few well-defined structures, loops can be forme ...
... bend in proline allow the polypeptide backbone to fold into a tight U shape. Turns allow large proteins to fold into highly compact structures. A polypeptide backbone also may contain longer bends, or loops. In contrast with turns, which exhibit just a few well-defined structures, loops can be forme ...
In Silico Prediction of Peroxisomal Proteins in Mouse
... The import of most proteins into the peroxisomal matrix is signal mediated. Almost all peroxisomal matrix proteins carry the type 1 (PTS1) signal at the extreme C-terminus, consisting of three amino acids, S/AKL. A few peroxisomal proteins contain type 2 (PTS2) targeting signal located near the N-te ...
... The import of most proteins into the peroxisomal matrix is signal mediated. Almost all peroxisomal matrix proteins carry the type 1 (PTS1) signal at the extreme C-terminus, consisting of three amino acids, S/AKL. A few peroxisomal proteins contain type 2 (PTS2) targeting signal located near the N-te ...
Chapter 3 Presentation: Macromolecules
... by H-bonds. • The β-pleated sheet forms when 2 polypeptides are aligned side by side and hydrogen bond along their lengths. ...
... by H-bonds. • The β-pleated sheet forms when 2 polypeptides are aligned side by side and hydrogen bond along their lengths. ...
PLANT PROTEINS FOR THE FUTURE-English
... feed. Amaranth and quinoa are considered “pseudocereals” and are also good sources of proteins. Amaranth seeds contain lysine, an essential amino acid, limited in other grains or plant sources but are limited in some essential amino acids, such as leucine and threonine. Amaranth seed may be a promis ...
... feed. Amaranth and quinoa are considered “pseudocereals” and are also good sources of proteins. Amaranth seeds contain lysine, an essential amino acid, limited in other grains or plant sources but are limited in some essential amino acids, such as leucine and threonine. Amaranth seed may be a promis ...
Biochemistry H Silent Tea Party Name_______________ 1. What is
... the substance in which the solute is dissolved within a solution 11. What is suspension? a mixture in which all of the components are not evenly mixed. The mixture can separate on standing. 12. What are macromolecules? ...
... the substance in which the solute is dissolved within a solution 11. What is suspension? a mixture in which all of the components are not evenly mixed. The mixture can separate on standing. 12. What are macromolecules? ...
Transcription/Translation Instructions
... 11) How many proteins did your group create? ____________________ 12) How many codons were contained in each of mRNA sequences that coded for a protein? Protein 1. ____________________ Protein 2. ____________________ Protein 3. ____________________ 13) How many amino acids were contained in each of ...
... 11) How many proteins did your group create? ____________________ 12) How many codons were contained in each of mRNA sequences that coded for a protein? Protein 1. ____________________ Protein 2. ____________________ Protein 3. ____________________ 13) How many amino acids were contained in each of ...
Proteins synthesisand expression
... extremely intricate shapes. • A haemoglobin molecule is formed for separate polypeptide chains. • It also has a haem group, which contains iron. • The inorganic group is known as the prosthetic group. • In haemoglobin it aids oxygen transport. ...
... extremely intricate shapes. • A haemoglobin molecule is formed for separate polypeptide chains. • It also has a haem group, which contains iron. • The inorganic group is known as the prosthetic group. • In haemoglobin it aids oxygen transport. ...
Carbohydrates – Complex (Polysaccharides)
... A typical tetrasaccharide linker (blue) connects a glycosamino-glycan—in this case chondroitin 4-sulfate (orange)—to a Ser residue (pink) in the core protein. The xylose residue at the reducing end of the linker is joined by its anomeric carbon to the hydroxyl of the Ser residue. ...
... A typical tetrasaccharide linker (blue) connects a glycosamino-glycan—in this case chondroitin 4-sulfate (orange)—to a Ser residue (pink) in the core protein. The xylose residue at the reducing end of the linker is joined by its anomeric carbon to the hydroxyl of the Ser residue. ...
Carbon compounds - Sonoma Valley High School
... • It can form single, double or triple bonds with other atoms. • Carbon is central to large, organic molecules • It is the ‘backbone’ of the molecule. Left side: what does ‘backbone’ mean in this context? ...
... • It can form single, double or triple bonds with other atoms. • Carbon is central to large, organic molecules • It is the ‘backbone’ of the molecule. Left side: what does ‘backbone’ mean in this context? ...
The six elements that make up 99.9% of all living things include
... 1. they are lipids 2. they will react with most body chemicals 3. they can only be used once 4. they usually slow down reactions and prevent overheating of the cells 5. they usually speed up chemical reactions ...
... 1. they are lipids 2. they will react with most body chemicals 3. they can only be used once 4. they usually slow down reactions and prevent overheating of the cells 5. they usually speed up chemical reactions ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.