Ch 2 - Biochemistry
... Irreversibly denatured proteins cannot refold and are formed by extreme pH or temperature change ...
... Irreversibly denatured proteins cannot refold and are formed by extreme pH or temperature change ...
A look at macromolecules (Text pages 38
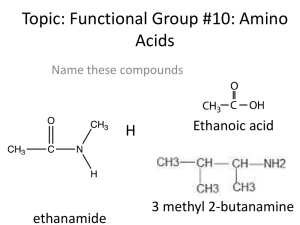
... Amino acids are the monomers that comprise proteins (proteins are poly-amino acids) • 20 common amino acids and a few unique to bacteria • all have some things in common o carboxylic acid end o amino end o can bond end to end via the Peptide Bond to form complex molecules with three dimensional char ...
... Amino acids are the monomers that comprise proteins (proteins are poly-amino acids) • 20 common amino acids and a few unique to bacteria • all have some things in common o carboxylic acid end o amino end o can bond end to end via the Peptide Bond to form complex molecules with three dimensional char ...
Chapter 15 - Translation of mRNA
... a. Studies involving T4 phage indicated that the genetic code is read in triplets b. Synthetic RNA helped to determine the genetic code c. The use of RNA copolymers and the triplet-binding assay also helped to crack the genetic code 4. Structure and function of tRNA a. The function of a tRNA depends ...
... a. Studies involving T4 phage indicated that the genetic code is read in triplets b. Synthetic RNA helped to determine the genetic code c. The use of RNA copolymers and the triplet-binding assay also helped to crack the genetic code 4. Structure and function of tRNA a. The function of a tRNA depends ...
Amino Acids Proteins, and Enzymes
... Involves interactions and cross-links between R groups in different areas of the peptide chain Is stabilized by: ...
... Involves interactions and cross-links between R groups in different areas of the peptide chain Is stabilized by: ...
Document
... In general, plants are relatively poor sources of protein. Animal foods (beef, poultry, seafood, dairy products, eggs) provide our best source for protein. Complementary proteins can be obtained by a varied diet. (These are proteins that individually are incomplete or low quality but when taken toge ...
... In general, plants are relatively poor sources of protein. Animal foods (beef, poultry, seafood, dairy products, eggs) provide our best source for protein. Complementary proteins can be obtained by a varied diet. (These are proteins that individually are incomplete or low quality but when taken toge ...
Presentazione di PowerPoint
... • occurrence of lignocellulosic fibers avoids the complete coagulation of proteins and facilitates processes like extrusion or injection-molding • mechanical properties of oil cake-based materials are lower than for similar starch-based composites but they possess a natural resistance to moisture th ...
... • occurrence of lignocellulosic fibers avoids the complete coagulation of proteins and facilitates processes like extrusion or injection-molding • mechanical properties of oil cake-based materials are lower than for similar starch-based composites but they possess a natural resistance to moisture th ...
Lecture 9 Protein Secondary Structure
... Protein Secondary Structure • Protein Structure • Protein Folding – Alpha helices, beta sheets, loops ...
... Protein Secondary Structure • Protein Structure • Protein Folding – Alpha helices, beta sheets, loops ...
Proteins - Structure, folding and domains
... in order to study the folding pathway one needs to look at kinetics (e.g. trpfluorescence by stopped-flow rapid mixing) e.g. phi-value analysis of mutants (Ferhst & co-workers) Range from 0 to 1 (effect of mutation on denatured or folded state). ...
... in order to study the folding pathway one needs to look at kinetics (e.g. trpfluorescence by stopped-flow rapid mixing) e.g. phi-value analysis of mutants (Ferhst & co-workers) Range from 0 to 1 (effect of mutation on denatured or folded state). ...
AP Biology 042 – Biological Molecules Video
... monomers together in a certain sequence/order they have a. The process of “putting monomers together” is called b. What is lost during the process of #11? c. What kind of bond is formed generally? Specifically between amino acids of a protein? d. What must be added to break the bonds? e. What is the ...
... monomers together in a certain sequence/order they have a. The process of “putting monomers together” is called b. What is lost during the process of #11? c. What kind of bond is formed generally? Specifically between amino acids of a protein? d. What must be added to break the bonds? e. What is the ...
RNA and Protein Synthesis
... nitrogen bases, (U vs. T), and the structure (single stranded vs. double helix.) 5. What are the three types of RNA and what is their function? Messenger RNA (mRNA)-Transcribes the code from DNA and takes it from the nucleus to the cytoplasm. Transfer RNA (tRNA)- Transfers amino acids from the cytop ...
... nitrogen bases, (U vs. T), and the structure (single stranded vs. double helix.) 5. What are the three types of RNA and what is their function? Messenger RNA (mRNA)-Transcribes the code from DNA and takes it from the nucleus to the cytoplasm. Transfer RNA (tRNA)- Transfers amino acids from the cytop ...
Life Substances
... What are their functions? What elements make uP Proteins? Define amino acids. How many amino acids are there? What makes one amino acid different from another? What do they look like? How are amino acids linked together? Define peptide bond What determines the kind of protein you have? Are hydrogen ...
... What are their functions? What elements make uP Proteins? Define amino acids. How many amino acids are there? What makes one amino acid different from another? What do they look like? How are amino acids linked together? Define peptide bond What determines the kind of protein you have? Are hydrogen ...
Getting things where they need to go: Protein Targeting
... Highly hydrophobic + charged - charged Hydroxylated Other ...
... Highly hydrophobic + charged - charged Hydroxylated Other ...
Organic Compounds
... • The 20 different amino acids can be joined by peptide bonds with an almost infinite number of combinations • Proteins vary greatly in size – some are as small as 3 amino acids in length – some are as large as 34350 amino acids in length • 4 levels of structural complexity in proteins • Primary str ...
... • The 20 different amino acids can be joined by peptide bonds with an almost infinite number of combinations • Proteins vary greatly in size – some are as small as 3 amino acids in length – some are as large as 34350 amino acids in length • 4 levels of structural complexity in proteins • Primary str ...
Chapter 3
... -speed up chemical reactions without becoming part of the reaction…thus, one enzyme can speed up thousands of chemical reactions. -called “catalysts” -lower the “activation energy” or the amount of energy that is needed to start a reaction. When a protein undergoes a shape change, it loses its abili ...
... -speed up chemical reactions without becoming part of the reaction…thus, one enzyme can speed up thousands of chemical reactions. -called “catalysts” -lower the “activation energy” or the amount of energy that is needed to start a reaction. When a protein undergoes a shape change, it loses its abili ...
Anatomy & Physiology
... the blood (hemoglobin) and across cell membranes Catalysts (Enzymes)-act as biological catalysts, to regulate and accelerate the rate of biochemical reactions without being used up in the process. ...
... the blood (hemoglobin) and across cell membranes Catalysts (Enzymes)-act as biological catalysts, to regulate and accelerate the rate of biochemical reactions without being used up in the process. ...
Biochemistry
... • They make up the structural parts of cells, enzymes, antibodies, hormones and membrane proteins. • Chemically they consist of an amine group (NH2) and a carboxyl group (COOH) and an “R” group. • There are 20 different R groups ...
... • They make up the structural parts of cells, enzymes, antibodies, hormones and membrane proteins. • Chemically they consist of an amine group (NH2) and a carboxyl group (COOH) and an “R” group. • There are 20 different R groups ...
lecture08_12
... • Generate a dataset of proteins with a common function (DNA binding protein) • Generate a control dataset • Calculate the different properties which are characteristic of the protein family you are interested for all the proteins in the data (DNA binding proteins and the non-DNA binding proteins • ...
... • Generate a dataset of proteins with a common function (DNA binding protein) • Generate a control dataset • Calculate the different properties which are characteristic of the protein family you are interested for all the proteins in the data (DNA binding proteins and the non-DNA binding proteins • ...
1.3.6 Structural Role of Biomolecules Worksheet
... Symptoms: the bones _______________and become weak – common in ____________ ...
... Symptoms: the bones _______________and become weak – common in ____________ ...
Cell Membrane Structure & Function
... – 1.Membrane selects what substances will enter – 2.Take up molecules present in high concentration – 3 Part of protein extends through bilayer – 4.May be non polar helix beta-pleated sheets of non polar amino acids – 5.Non polar portion held within interior of bilayer – 6.Polar ends protrude from b ...
... – 1.Membrane selects what substances will enter – 2.Take up molecules present in high concentration – 3 Part of protein extends through bilayer – 4.May be non polar helix beta-pleated sheets of non polar amino acids – 5.Non polar portion held within interior of bilayer – 6.Polar ends protrude from b ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.