Daily Essential Electrolytes, Protein, and Probiotics
... that build or tear down body tissue. Manganese is used by enzymes needed to utilize several vitamins. Zinc is used in over 80 enzyme reactions that are critical for life in all people! Another example is that of sodium. Unlike table salt or sodium chloride, bio-organic sodium, i.e., sodium carried n ...
... that build or tear down body tissue. Manganese is used by enzymes needed to utilize several vitamins. Zinc is used in over 80 enzyme reactions that are critical for life in all people! Another example is that of sodium. Unlike table salt or sodium chloride, bio-organic sodium, i.e., sodium carried n ...
The Pentatricopeptide Repeat Protein OTP87 Is Essential for RNA
... • Arabidopsis thaliana – most favored model for plant biology because of its genetics A small dicot in the mustard family ...
... • Arabidopsis thaliana – most favored model for plant biology because of its genetics A small dicot in the mustard family ...
1-Three states of matter . A: density, volume and weight B: solid
... 19. Compounds with an amino group (-NH2) on one end and a carboxyl group (COOH) on the other end are known as _______________ (Hint: They are the building blocks of proteins). A. nucleic acids B. amino acids C. carbohydrates D. lipids 20. ________________ are macromolecules that contain carbon, hyd ...
... 19. Compounds with an amino group (-NH2) on one end and a carboxyl group (COOH) on the other end are known as _______________ (Hint: They are the building blocks of proteins). A. nucleic acids B. amino acids C. carbohydrates D. lipids 20. ________________ are macromolecules that contain carbon, hyd ...
Daily Essential Electrolytes, Protein, and Probiotics
... that build or tear down body tissue. Manganese is used by enzymes needed to utilize several vitamins. Zinc is used in over 80 enzyme reactions that are critical for life in all people! Another example is that of sodium. Unlike table salt or sodium chloride, bio-organic sodium, i.e., sodium carried n ...
... that build or tear down body tissue. Manganese is used by enzymes needed to utilize several vitamins. Zinc is used in over 80 enzyme reactions that are critical for life in all people! Another example is that of sodium. Unlike table salt or sodium chloride, bio-organic sodium, i.e., sodium carried n ...
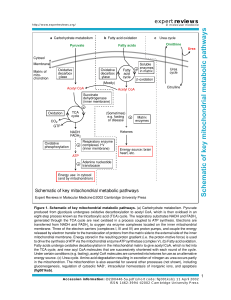
Schematic of key mitochondrial metabolic pathways
... transferred from NADH and FADH2 to oxygen via enzyme complexes located on the inner mitochondrial membrane. Three of the electron carriers (complexes I, III and IV) are proton pumps, and couple the energy released by electron transfer to the translocation of protons from the matrix side to the exter ...
... transferred from NADH and FADH2 to oxygen via enzyme complexes located on the inner mitochondrial membrane. Three of the electron carriers (complexes I, III and IV) are proton pumps, and couple the energy released by electron transfer to the translocation of protons from the matrix side to the exter ...
PDF File
... of both substrate and potential ribozyme ligands can determine whether such groups are indeed ligands for particular metal ions (5, 17, 27). Possible complications that may arise in these analyses, but that do not apply to the Tetrahymena ribozyme, are described in the Supporting Information. Consid ...
... of both substrate and potential ribozyme ligands can determine whether such groups are indeed ligands for particular metal ions (5, 17, 27). Possible complications that may arise in these analyses, but that do not apply to the Tetrahymena ribozyme, are described in the Supporting Information. Consid ...
Carbon Compounds - Model High School
... Broken down by the digestive system into amino acids which are ___________ then absorbed into the body through the bloodstream, where the body cells take the amino acids and makes proteins for muscles. ...
... Broken down by the digestive system into amino acids which are ___________ then absorbed into the body through the bloodstream, where the body cells take the amino acids and makes proteins for muscles. ...
Second test - rci.rutgers.edu
... A. it is sensitive to voltage B. it becomes inactivated spontaneously C. it consists of seven hydrophobic transmembrane segments D. it is much more permeable to Na+ than to K+ E. none of the above ...
... A. it is sensitive to voltage B. it becomes inactivated spontaneously C. it consists of seven hydrophobic transmembrane segments D. it is much more permeable to Na+ than to K+ E. none of the above ...
svhs lab bioogy - Sonoma Valley High School
... A) Be able to explain atomic structure, and how atoms combine to create molecules. Describe ionic and covalent bonding. Know the energy levels of the 3 states of matter. (Page 54) B) Be able to explain the structure of the water molecule, why it is said to be polar, and the characteristics due to it ...
... A) Be able to explain atomic structure, and how atoms combine to create molecules. Describe ionic and covalent bonding. Know the energy levels of the 3 states of matter. (Page 54) B) Be able to explain the structure of the water molecule, why it is said to be polar, and the characteristics due to it ...
chemistry advanced may 2010 marking scheme
... (b) Complete the diagram to show the conical flask intended to collect the two-layer mixture. Show clearly whether this flask connects tightly, or otherwise, with the rest of the apparatus. If the flask is shown connected firmly to the adaptor, award no mark. (c) (i) Explain why the distillate consi ...
... (b) Complete the diagram to show the conical flask intended to collect the two-layer mixture. Show clearly whether this flask connects tightly, or otherwise, with the rest of the apparatus. If the flask is shown connected firmly to the adaptor, award no mark. (c) (i) Explain why the distillate consi ...
Lecture notes Chapter 22-23
... have positive and negative charges. The attraction of the oppositely charged side chains forms a strong bond called a salt bridge. If the pH changes, the basic and acidic side chains lose their ionic charges and cannot form salt bridge, which causes a change in the shape of the protein. 5. Disulfide ...
... have positive and negative charges. The attraction of the oppositely charged side chains forms a strong bond called a salt bridge. If the pH changes, the basic and acidic side chains lose their ionic charges and cannot form salt bridge, which causes a change in the shape of the protein. 5. Disulfide ...
Chemistry Final Exam Review 2006-2007
... d. oxygen molecule, how many unshared electron pairs 2. Ionic compounds generally form: surround the carbon? a. Liquids a. 2 b. Gases b. 0 c. Crystals c. 8 d. molecules d. 4 3. In metallic bonding, the valence electrons of all 12. In nonpolar covalent bonds, valence electrons are atoms are shared in ...
... d. oxygen molecule, how many unshared electron pairs 2. Ionic compounds generally form: surround the carbon? a. Liquids a. 2 b. Gases b. 0 c. Crystals c. 8 d. molecules d. 4 3. In metallic bonding, the valence electrons of all 12. In nonpolar covalent bonds, valence electrons are atoms are shared in ...
Transgenic Approach for Abiotic Stress Tolerance
... locations, these proteins are mostly the enzymes involved in diverse functions such as production of different osmolytes, protein degradation, signal transduction events, gene regulation and transport. Roles of some WSPs is not well defined (i.e. such as for dehydrins, late embryogenesis, abundant p ...
... locations, these proteins are mostly the enzymes involved in diverse functions such as production of different osmolytes, protein degradation, signal transduction events, gene regulation and transport. Roles of some WSPs is not well defined (i.e. such as for dehydrins, late embryogenesis, abundant p ...
Summary of 5.4
... polyethenol which dissolves due to hydrogen bonds formed between the polymer molecules and the water molecules. 5.4.2i Amino acids 5.4.2i describe and carry out, where appropriate, experiments to investigate the characteristic behaviour of amino acids. This is limited to: i acidity and basicity and ...
... polyethenol which dissolves due to hydrogen bonds formed between the polymer molecules and the water molecules. 5.4.2i Amino acids 5.4.2i describe and carry out, where appropriate, experiments to investigate the characteristic behaviour of amino acids. This is limited to: i acidity and basicity and ...
Net Ionic Prep Session NMSI INSTRUCTOR
... ALL THREE. Spend 3 minutes writing products using the solubility rules and strong acidbase guidelines listed on the other side of this page. To get the easy three points involved with step SIX above do the following: Write the reactants. On the product side, open a set of brackets [ ]. Put the metal ...
... ALL THREE. Spend 3 minutes writing products using the solubility rules and strong acidbase guidelines listed on the other side of this page. To get the easy three points involved with step SIX above do the following: Write the reactants. On the product side, open a set of brackets [ ]. Put the metal ...
Experiment 4 - Chemistry| |UCC
... When hard water is heated, CaCO3 precipitates out, which then clogs pipes and industrial boilers. This leads to malfunction or damage and is expensive to remove ...
... When hard water is heated, CaCO3 precipitates out, which then clogs pipes and industrial boilers. This leads to malfunction or damage and is expensive to remove ...
Name ______Mr. Perfect_______________________________
... 1. If the n quantum number of an atomic orbital is equal to 4, what are the possible values of l ? What are the possible values of ml if the quantum number l is equal to 1? (5 pts) l ranges from 0 to n-1 ...
... 1. If the n quantum number of an atomic orbital is equal to 4, what are the possible values of l ? What are the possible values of ml if the quantum number l is equal to 1? (5 pts) l ranges from 0 to n-1 ...
Transport
... body of complex chemical compounds from smaller simpler compounds (e.g., proteins from amino acids), usually with the use of energy. ...
... body of complex chemical compounds from smaller simpler compounds (e.g., proteins from amino acids), usually with the use of energy. ...
A Chromium(III)−Superoxo Complex in Oxygen Atom Transfer
... intermediate in the oxidation of cysteine in CDO (see Schemes 1 and 2). The Cr(III)−superoxo complex, [CrIII(O2)(TMC)(Cl)]+ (1), was generated by bubbling O2 through a solution of [CrII(TMC)(Cl)]+ in CH3CN at −10 °C, as reported previously.10 The intermediate 1 was metastable so that it could be use ...
... intermediate in the oxidation of cysteine in CDO (see Schemes 1 and 2). The Cr(III)−superoxo complex, [CrIII(O2)(TMC)(Cl)]+ (1), was generated by bubbling O2 through a solution of [CrII(TMC)(Cl)]+ in CH3CN at −10 °C, as reported previously.10 The intermediate 1 was metastable so that it could be use ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.