AQA GCSE Chemistry My Revision Notes
... (a) Suggest one reason why this part of Newlands’ table is different from the modern one. (1 mark) In 1869 Dimitri Mendeleev arranged the elements by putting them in order of their atomic weights. When he put them into a table he ensured that elements with similar properties were in columns. (b) Wha ...
... (a) Suggest one reason why this part of Newlands’ table is different from the modern one. (1 mark) In 1869 Dimitri Mendeleev arranged the elements by putting them in order of their atomic weights. When he put them into a table he ensured that elements with similar properties were in columns. (b) Wha ...
Reactions in Aqueous Solution (Brown 13th-Fossum
... • An oxidation occurs when an atom or ion loses electrons. • A reduction occurs when an atom or ion gains electrons. • One cannot occur without the other. Combustion reactions and corrosion (rusting of metals) are two examples. To determine if an oxidation-reduction reaction has occurred, we assign ...
... • An oxidation occurs when an atom or ion loses electrons. • A reduction occurs when an atom or ion gains electrons. • One cannot occur without the other. Combustion reactions and corrosion (rusting of metals) are two examples. To determine if an oxidation-reduction reaction has occurred, we assign ...
Energy Releasing Pathway
... Liberates H+ and NAD+ steals the electrons from H+ to form NADH + H +. The hole left by the leaving H+ is backfilled by Pi. This step balances the G3P with a P on either end. This happens twice or once for each G3P. How many NADH + H+ are formed per glucose? ...
... Liberates H+ and NAD+ steals the electrons from H+ to form NADH + H +. The hole left by the leaving H+ is backfilled by Pi. This step balances the G3P with a P on either end. This happens twice or once for each G3P. How many NADH + H+ are formed per glucose? ...
$doc.title
... Pep.de Synthesis • Pep.de synthesis requires that different amide bonds must be formed in a desired sequence • The growing chain is protected at the carboxyl terminal and added amino acids are N-‐pro ...
... Pep.de Synthesis • Pep.de synthesis requires that different amide bonds must be formed in a desired sequence • The growing chain is protected at the carboxyl terminal and added amino acids are N-‐pro ...
June review January 2012 part A
... (l) A neutral nucleus is surrounded by one or more negatively charged electrons. (2) A neutral nucleus is surrounded by one or more positively charged electrons. (3) A positively charged nucleus is surrounded by one or more negatively charged electrons. (4) A positively charged nucleus is surrounded ...
... (l) A neutral nucleus is surrounded by one or more negatively charged electrons. (2) A neutral nucleus is surrounded by one or more positively charged electrons. (3) A positively charged nucleus is surrounded by one or more negatively charged electrons. (4) A positively charged nucleus is surrounded ...
The Cell in Motion
... have severe symptoms like mental retardation. This example illustrates how a gene is linked to a disease. ...
... have severe symptoms like mental retardation. This example illustrates how a gene is linked to a disease. ...
Synthesis and Characterization of PhCC-Ru-L-Ru
... Introduction Many research groups have investigated the ability of transition metal complexes to transfer electrons from both their ground and electronically exited states.1 One particularly interesting result is the use of ruthenium-based chromphores to convert light energy into electrical energy o ...
... Introduction Many research groups have investigated the ability of transition metal complexes to transfer electrons from both their ground and electronically exited states.1 One particularly interesting result is the use of ruthenium-based chromphores to convert light energy into electrical energy o ...
CHAP 1 - NCERT books
... while SO2 and SO3 are gases. Decomposition of calcium carbonate to calcium oxide and carbon dioxide on heating is an important decomposition reaction used in various industries. Calcium oxide is called lime or quick lime. It has many uses – one is in the manufacture of cement. When a decomposition r ...
... while SO2 and SO3 are gases. Decomposition of calcium carbonate to calcium oxide and carbon dioxide on heating is an important decomposition reaction used in various industries. Calcium oxide is called lime or quick lime. It has many uses – one is in the manufacture of cement. When a decomposition r ...
Molecular Insight into Hydrothermal Solution Geochemistry
... Aqueous fluids under extreme conditions of temperature and pressure occur ubiquitously throughout the Earth’s crust and upper mantle. Throughout geological time, they have been and still are responsible for the transport of enormous amounts of heat and chemical mass within the crust. They discharge ...
... Aqueous fluids under extreme conditions of temperature and pressure occur ubiquitously throughout the Earth’s crust and upper mantle. Throughout geological time, they have been and still are responsible for the transport of enormous amounts of heat and chemical mass within the crust. They discharge ...
Chemical Reactions
... 1. Fe2O3 + H2 Fe + H 2O Compare the number of each atom in the reactants to the 2. Fe 21 number of the same atom in the 1. O 31 product 2. H 22 Pick one of the unequal atoms 3.Fe2O3 + H2 2Fe and multiply the compound by + H 2O a number so that the atoms are Write the skeleton equation ...
... 1. Fe2O3 + H2 Fe + H 2O Compare the number of each atom in the reactants to the 2. Fe 21 number of the same atom in the 1. O 31 product 2. H 22 Pick one of the unequal atoms 3.Fe2O3 + H2 2Fe and multiply the compound by + H 2O a number so that the atoms are Write the skeleton equation ...
PACK 3 - Speyside High School
... Some chemical reactions are Anabolic and involve the building of complex molecular structures from simpler ones - eg Protein synthesis; amino acid synthesis. These reactions are Endergonic - they need energy. Living things have the ability to link these energy consuming and energy producing reaction ...
... Some chemical reactions are Anabolic and involve the building of complex molecular structures from simpler ones - eg Protein synthesis; amino acid synthesis. These reactions are Endergonic - they need energy. Living things have the ability to link these energy consuming and energy producing reaction ...
Amino Acids Are the Building Blocks Of Proteins
... 1. Construct two separate amino acids using the Molymod® atoms, covalent bonds and hydrogen bonds. a. Identify the following components: amino group, carboxyl group, the R group or sidechain, alpha carbon, carboxyl carbon, nitrogen. (See labeled diagram and parts list above.) b. Compare the two amin ...
... 1. Construct two separate amino acids using the Molymod® atoms, covalent bonds and hydrogen bonds. a. Identify the following components: amino group, carboxyl group, the R group or sidechain, alpha carbon, carboxyl carbon, nitrogen. (See labeled diagram and parts list above.) b. Compare the two amin ...
Copper(II) Mixed Ligands Complexes of Hydroxamic Acids with
... occupy an axial position. The importance of the CuII histidine co-ordination to explain the transport of copper in the human plasma has led to a series of investigations (involving potentiometry, spectroscopy in the UV, visible, infrared and Raman, calorimetry, X-ray and EPR) attempting to elucidate ...
... occupy an axial position. The importance of the CuII histidine co-ordination to explain the transport of copper in the human plasma has led to a series of investigations (involving potentiometry, spectroscopy in the UV, visible, infrared and Raman, calorimetry, X-ray and EPR) attempting to elucidate ...
Copper(II) Complexes with New Polypodal Ligands Presenting Axial
... ferromagnetic to antiferromagnetic have been reported,6-9 the ferromagnetic interaction in polynuclear copper(II) complexes being comparatively rare.1 Several types of ligand systems that can bind two metal ions in close proximity have been studied due to their interesting catalytic properties and t ...
... ferromagnetic to antiferromagnetic have been reported,6-9 the ferromagnetic interaction in polynuclear copper(II) complexes being comparatively rare.1 Several types of ligand systems that can bind two metal ions in close proximity have been studied due to their interesting catalytic properties and t ...
Concept Sheet for Semester 2 material - mvhs
... tension – cohesion model; transpiration, guard cells (and how they close and open), water potential, symplast versus apoplast pathways Translocation in phloem – pressure-flow hypothesis, role of active transport and osmosis in loading at source and unloading at sink cells Tropisms – to light (where ...
... tension – cohesion model; transpiration, guard cells (and how they close and open), water potential, symplast versus apoplast pathways Translocation in phloem – pressure-flow hypothesis, role of active transport and osmosis in loading at source and unloading at sink cells Tropisms – to light (where ...
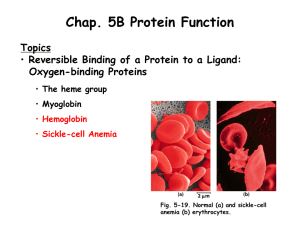
Chapter 5B Lecture
... different parts of a protein. The binding sites of an allosteric protein typically consist of stable segments in proximity to relatively unstable segments, with the later capable of frequent changes in conformation or intrinsic disorder (Fig. 5-13). When a ligand binds, the moving parts of the prote ...
... different parts of a protein. The binding sites of an allosteric protein typically consist of stable segments in proximity to relatively unstable segments, with the later capable of frequent changes in conformation or intrinsic disorder (Fig. 5-13). When a ligand binds, the moving parts of the prote ...
Amino Acids are the Building Blocks of Proteins
... 1. Construct two separate amino acids using the Molymod® atoms, covalent bonds and hydrogen bonds. a. Identify the following components: amino group, carboxyl group, the R group or sidechain, alpha carbon, carboxyl carbon, nitrogen. (See labeled diagram and parts list above.) b. Compare the two amin ...
... 1. Construct two separate amino acids using the Molymod® atoms, covalent bonds and hydrogen bonds. a. Identify the following components: amino group, carboxyl group, the R group or sidechain, alpha carbon, carboxyl carbon, nitrogen. (See labeled diagram and parts list above.) b. Compare the two amin ...
Module 3 Transition elements - Pearson Schools and FE Colleges
... When white light passes through a solution containing transition metal ions, some of the wavelengths of visible light are absorbed. The colour that we observe is a mixture of the wavelengths of light that have not been absorbed. For example, a solution of copper(II) sulfate appears pale blue because ...
... When white light passes through a solution containing transition metal ions, some of the wavelengths of visible light are absorbed. The colour that we observe is a mixture of the wavelengths of light that have not been absorbed. For example, a solution of copper(II) sulfate appears pale blue because ...
1. PROTEIN MODIFICATION 1.1 What are posttranslational
... Describe protein degradation by ubiquitinylation. How is ubiquitin connected to a protein? ...
... Describe protein degradation by ubiquitinylation. How is ubiquitin connected to a protein? ...
Photosynthesis
... Temperature- as you increase temperature, enzyme action will increase until an opitmum temperature of 37 degrees Celsius is reached Enzyme-Substrate Concentration1. High levels of enzyme + low levels of substrate = an increase in enzyme action 2. Low levels of enzyme + high levels of substrate = a d ...
... Temperature- as you increase temperature, enzyme action will increase until an opitmum temperature of 37 degrees Celsius is reached Enzyme-Substrate Concentration1. High levels of enzyme + low levels of substrate = an increase in enzyme action 2. Low levels of enzyme + high levels of substrate = a d ...
The stabilization is only possible if the planes defined by the sp2
... If the Φ and Ψ angles are constant throughout a polypeptide chain, the polypeptide will have the shape of a helix. A given set of Φ and Ψ angles will determine the type of helix, i.e., n, the number a.a. per turn, p, the pitch, and d, the distance traversed by one a.a. along the helix axis. The hand ...
... If the Φ and Ψ angles are constant throughout a polypeptide chain, the polypeptide will have the shape of a helix. A given set of Φ and Ψ angles will determine the type of helix, i.e., n, the number a.a. per turn, p, the pitch, and d, the distance traversed by one a.a. along the helix axis. The hand ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.