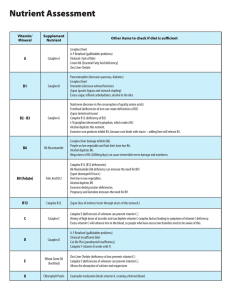
Chapter 1: Matter and Measurement
... Propane, gasoline, kerosene, oil are fuels. Drugs and plastics are produced by chemical industries. Carbon atoms form chains and rings to which other atoms are attached and act as the framework of molecules. All organic compounds contain C atoms; almost all contain H atoms; and many common one ...
... Propane, gasoline, kerosene, oil are fuels. Drugs and plastics are produced by chemical industries. Carbon atoms form chains and rings to which other atoms are attached and act as the framework of molecules. All organic compounds contain C atoms; almost all contain H atoms; and many common one ...
Cellular Respiration notes
... Electron Transport Chain • The electron transport chain is a series of chemical reactions ending with hydrogen combining with oxygen to form water. Carbon dioxide is released as a waste product as it is formed in several stages of the Krebs cycle. • Each reaction produces a small amount of energy, ...
... Electron Transport Chain • The electron transport chain is a series of chemical reactions ending with hydrogen combining with oxygen to form water. Carbon dioxide is released as a waste product as it is formed in several stages of the Krebs cycle. • Each reaction produces a small amount of energy, ...
STRUCTURE AND FUNCTION
... biologically usable form, ammonia. This process is done by a special group of organisms called nitrogen fixing bacteria, which convert the atmospheric nitrogen into ammonia through nitrogen fixation. These nitrogen fixing bacteria are essential to the persistence of life on this planet because they ...
... biologically usable form, ammonia. This process is done by a special group of organisms called nitrogen fixing bacteria, which convert the atmospheric nitrogen into ammonia through nitrogen fixation. These nitrogen fixing bacteria are essential to the persistence of life on this planet because they ...
3 Amino acids and crude protein - DLG
... retention, in individual cases over as well as underestimation possible when reference animals are not adequate, this especially with small scaling of weights ...
... retention, in individual cases over as well as underestimation possible when reference animals are not adequate, this especially with small scaling of weights ...
Amino acids - Zanichelli online
... What Are the Chemical Structures and Functions of Carbohydrates? ...
... What Are the Chemical Structures and Functions of Carbohydrates? ...
Lab 8 - Electrophoresis
... protein does not migrate in an electric field is called the isoelectric point. Most neutral amino acids have isoelectric points around pH 6.0. The isoelectric points of aspartic acid and glutamic acid, however, are close to pH 3. Therefore, at pH 6, these acidic amino acids carry a negative charge a ...
... protein does not migrate in an electric field is called the isoelectric point. Most neutral amino acids have isoelectric points around pH 6.0. The isoelectric points of aspartic acid and glutamic acid, however, are close to pH 3. Therefore, at pH 6, these acidic amino acids carry a negative charge a ...
PPT File
... • The four carbons in the four CO2 molecules plus the two carbons in the two CO2 molecules from the prep step are evidence that the initial six carbon sugar molecule is ...
... • The four carbons in the four CO2 molecules plus the two carbons in the two CO2 molecules from the prep step are evidence that the initial six carbon sugar molecule is ...
chapter_5_Mod_2009
... enzyme-substrate complex is formed. This destabilizes the bonds in the substrate, speeding up the reaction. ...
... enzyme-substrate complex is formed. This destabilizes the bonds in the substrate, speeding up the reaction. ...
4 Cell Resp Part 2 NT
... yields ____________________________________ only in presence of O2 (__________________________________________) ...
... yields ____________________________________ only in presence of O2 (__________________________________________) ...
Origins of Sugars in the Prebiotic World
... • If the nucleophile is the 3’-OH group of another NTP, then a nucleic acid is generated: polymer of nucleotides – Oligomers (“oligos”) short length (DNA/RNA polymers of long ...
... • If the nucleophile is the 3’-OH group of another NTP, then a nucleic acid is generated: polymer of nucleotides – Oligomers (“oligos”) short length (DNA/RNA polymers of long ...
Full_ppt_ch20
... pH and Ionization • Acidic amino acids such as aspartic acid have a second carboxyl group that can donate and accept protons – Amino acids with ionizable side chains have 4 forms in solution • -Cys, Tyr, Lys, Arg, His, Asp, Glu ...
... pH and Ionization • Acidic amino acids such as aspartic acid have a second carboxyl group that can donate and accept protons – Amino acids with ionizable side chains have 4 forms in solution • -Cys, Tyr, Lys, Arg, His, Asp, Glu ...
15-25 kD
... – Class – Order – Family – Genus – Species • Traditional classification based upon traits: – Morphological – Behavioral ...
... – Class – Order – Family – Genus – Species • Traditional classification based upon traits: – Morphological – Behavioral ...
hat is Ideal Protein - Herndon-reston
... Cataplex A (vitamin A is needed with calcium to prevent kidney stones.) Cataplex C (without vitamin C, bones can become decalcified.) Cataplex D (without vitamin D, calcium doesn’t get absorbed in the intestines.) Cataplex E (without vitamin E and F, calcium doesn’t get absorbed in the nerves and mu ...
... Cataplex A (vitamin A is needed with calcium to prevent kidney stones.) Cataplex C (without vitamin C, bones can become decalcified.) Cataplex D (without vitamin D, calcium doesn’t get absorbed in the intestines.) Cataplex E (without vitamin E and F, calcium doesn’t get absorbed in the nerves and mu ...
AMINO ACIDS I. Function of amino acids A. Building blocks of
... R-COOH <--------> R-COO- + H+ R-NH3+ <---------> R-NH2 + H+ The equilibrium reactions, as written, demonstrate that amino acids contain at least two weakly acidic groups. However, the carboxyl group is a far stronger acid than the amino group. At physiological pH (around 7.4) the carboxyl group will ...
... R-COOH <--------> R-COO- + H+ R-NH3+ <---------> R-NH2 + H+ The equilibrium reactions, as written, demonstrate that amino acids contain at least two weakly acidic groups. However, the carboxyl group is a far stronger acid than the amino group. At physiological pH (around 7.4) the carboxyl group will ...
No Slide Title - Palm Beach State College
... • Molecules—chemical particles composed of two or more atoms united by a chemical bond • Compounds—molecules composed of two or more different elements • Molecular formula—identifies constituent elements and how many atoms of each are present ...
... • Molecules—chemical particles composed of two or more atoms united by a chemical bond • Compounds—molecules composed of two or more different elements • Molecular formula—identifies constituent elements and how many atoms of each are present ...
Ch. 9 Cellular Respiration
... Function as enzymes directing the flow of reactions that move e(alternate between oxidized and reduced state) NADH and FADH2 are from Krebs and glycolysis NADH and FADH2 release H to these reactions H is split into H+ and eThe e- move through the carriers to the biggest e- acceptor (moving down hill ...
... Function as enzymes directing the flow of reactions that move e(alternate between oxidized and reduced state) NADH and FADH2 are from Krebs and glycolysis NADH and FADH2 release H to these reactions H is split into H+ and eThe e- move through the carriers to the biggest e- acceptor (moving down hill ...
Mutagenesis of human papillomavirus types 6 and 16 E7 open
... critical determinant of the characteristic migration of the HPV-6 and -16 E7 proteins. The change in the electrophoretic behaviour of the mutated proteins may be related to the negative charge of aspartic acid and the positive charge of arginine. Inspection of the first 10 residues of each protein r ...
... critical determinant of the characteristic migration of the HPV-6 and -16 E7 proteins. The change in the electrophoretic behaviour of the mutated proteins may be related to the negative charge of aspartic acid and the positive charge of arginine. Inspection of the first 10 residues of each protein r ...
Isomerism
... Butane CH3CH2CH2CH3 and methyl propane CH3CH(CH3)CH3 are chain isomers, they have different carbon chains. This is also known as branch isomerism. 1-bromo butane CH3 CH2CH2CH2Br and 2-bromo butane CH3CH2CHBrCH3 are positional isomers. They differ in the position of an atom or group (here the Br atom ...
... Butane CH3CH2CH2CH3 and methyl propane CH3CH(CH3)CH3 are chain isomers, they have different carbon chains. This is also known as branch isomerism. 1-bromo butane CH3 CH2CH2CH2Br and 2-bromo butane CH3CH2CHBrCH3 are positional isomers. They differ in the position of an atom or group (here the Br atom ...
Medical Biochemistry
... Animal cells contain alcohol dehydrogenase (ADH) which oxidizes ethanol to acetaldehyde. Acetaldehyde is oxidized to acetate by acetaldehyde dehydrogenase (AcDH). Acetaldehyde and acetate are toxic leading to the many side effects (the hangover) that are associated with alcohol consumption. The ADH ...
... Animal cells contain alcohol dehydrogenase (ADH) which oxidizes ethanol to acetaldehyde. Acetaldehyde is oxidized to acetate by acetaldehyde dehydrogenase (AcDH). Acetaldehyde and acetate are toxic leading to the many side effects (the hangover) that are associated with alcohol consumption. The ADH ...
bioinfo4
... ----------------------------------------------------Note the common offset for the 3 amino acids c,s and p A possible alignment is thus quickly found protein 1 n c s p t a ...
... ----------------------------------------------------Note the common offset for the 3 amino acids c,s and p A possible alignment is thus quickly found protein 1 n c s p t a ...
Multiple Choice Practice. A) P B) S C) Cl D) Li E) 1 F 1. Has the
... mole of I2 formed by this half-reaction? A) 2 B) 6 C) 8 D) 10 E) 12 30. Which of the following is always true at the triple point of a pure substance? A) The vapor pressure of the solid phase equals the vapor pressure of the liquid phase B) The temperature is 0.01K lower than the normal melting poi ...
... mole of I2 formed by this half-reaction? A) 2 B) 6 C) 8 D) 10 E) 12 30. Which of the following is always true at the triple point of a pure substance? A) The vapor pressure of the solid phase equals the vapor pressure of the liquid phase B) The temperature is 0.01K lower than the normal melting poi ...
File - Hoblitzell`s Science Spot
... 44. When comparing the digestion and absorption of proteins with fats it is important to know that: a. proteins are broken down into individual amino acids and absorbed by all intestinal cells at an equal rate. b. larger peptide molecules must be completely broken down into amino acids to be absorbe ...
... 44. When comparing the digestion and absorption of proteins with fats it is important to know that: a. proteins are broken down into individual amino acids and absorbed by all intestinal cells at an equal rate. b. larger peptide molecules must be completely broken down into amino acids to be absorbe ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.