Chapter 6 Proteins & Amino Acids
... Amino acids contain carbon, hydrogen, oxygen, nitrogen, and sometimes sulfur Unique amino acids consist of a central carbon with a carboxyl group, a hydrogen, a nitrogen-containing amine group, and a unique side chain There are 20 side chains and 20 unique amino acids • 9 essential amino acids • 11 ...
... Amino acids contain carbon, hydrogen, oxygen, nitrogen, and sometimes sulfur Unique amino acids consist of a central carbon with a carboxyl group, a hydrogen, a nitrogen-containing amine group, and a unique side chain There are 20 side chains and 20 unique amino acids • 9 essential amino acids • 11 ...
Equation Writing Information
... On the AP examination you will encounter a question in which you will be required to write net ionic equations for various reactions. In past years, students have been required to choose 5 of 8 reactions. Some of the reactions you will undoubtedly recognize; others you will not! Hopefully, at least ...
... On the AP examination you will encounter a question in which you will be required to write net ionic equations for various reactions. In past years, students have been required to choose 5 of 8 reactions. Some of the reactions you will undoubtedly recognize; others you will not! Hopefully, at least ...
Atomic Masses: Counting Atoms by Weighing
... To determine the number of oxygen molecules required, we must know how many carbon atoms are present in the pile of carbon. But individual atoms are far too small to see. We must learn to count atoms by weighing samples containing large numbers of them. In the last section we saw that we can easily ...
... To determine the number of oxygen molecules required, we must know how many carbon atoms are present in the pile of carbon. But individual atoms are far too small to see. We must learn to count atoms by weighing samples containing large numbers of them. In the last section we saw that we can easily ...
Biology-1 Exam Two You can write on this exam. Please put a W at
... 57. The transfer of a phosphate group to another molecule or compound is called phosphorylation. (T/F) 58. When an enzyme catalyzes a reaction, it raises the activation energy so that the reaction proceeds faster. (T/F) 59. In photosynthesis, for every 3 molecule of CO2 that enter the Calvin cycle, ...
... 57. The transfer of a phosphate group to another molecule or compound is called phosphorylation. (T/F) 58. When an enzyme catalyzes a reaction, it raises the activation energy so that the reaction proceeds faster. (T/F) 59. In photosynthesis, for every 3 molecule of CO2 that enter the Calvin cycle, ...
HW #2
... Give an implementation for BP DEC. You may use any of the modules we’ve discussed in class in your design. (b) Trios of base pairs (or codons) can be interpreted as codewords for particular amino acids. The table below shows the codon to amino acid mappings. Note that (1) the code is redundant, i.e. ...
... Give an implementation for BP DEC. You may use any of the modules we’ve discussed in class in your design. (b) Trios of base pairs (or codons) can be interpreted as codewords for particular amino acids. The table below shows the codon to amino acid mappings. Note that (1) the code is redundant, i.e. ...
Lecture 5
... A chemical reaction involves bond breaking and bond forming. When a reaction rearranges the atoms of molecules, existing bonds in the reactants must be broken and the new bonds of products formed. These processes require exchanges of energy between the mixture of molecules and surrounding environmen ...
... A chemical reaction involves bond breaking and bond forming. When a reaction rearranges the atoms of molecules, existing bonds in the reactants must be broken and the new bonds of products formed. These processes require exchanges of energy between the mixture of molecules and surrounding environmen ...
CARBOHYDRATES
... amount of energy stored in each organic molecule. – Carbohydrates, lipids and proteins all have a caloric value. ...
... amount of energy stored in each organic molecule. – Carbohydrates, lipids and proteins all have a caloric value. ...
(a) (b) - My SMCC
... • Instructions for making proteins are contained primarily in the DNA in the nucleus of the cell ...
... • Instructions for making proteins are contained primarily in the DNA in the nucleus of the cell ...
bbr052online 329..336 - Oxford Academic
... explore the MSA text file to search the highly conserved positions along the alignment. These conserved positions can be highlighted by colouring according to different criteria, such as percentage of identity. The residues showing whole conservation in the expanded set of sequences used for MSA wil ...
... explore the MSA text file to search the highly conserved positions along the alignment. These conserved positions can be highlighted by colouring according to different criteria, such as percentage of identity. The residues showing whole conservation in the expanded set of sequences used for MSA wil ...
Document
... – is the major protein of human plasma (3.4-4.7 g/dL) – Approximately 40% of albumin is present in plasma and the other 60% in the extracellular space – It synthesized in the liver as preproprotein – The synthesis of albumin is depressed in a variety of diseases, particularly those of the liver (dec ...
... – is the major protein of human plasma (3.4-4.7 g/dL) – Approximately 40% of albumin is present in plasma and the other 60% in the extracellular space – It synthesized in the liver as preproprotein – The synthesis of albumin is depressed in a variety of diseases, particularly those of the liver (dec ...
prepex3
... 3. Classify the reactions. Are you adding phosphate, are you oxidizing an –OH group, are you replacing an amino group, etc. 4. Identify the cofactor or other substrates that may be needed for that step. Oxidations require NAD+, reductions often require NADPH, amino additions require an –NH2 donor, p ...
... 3. Classify the reactions. Are you adding phosphate, are you oxidizing an –OH group, are you replacing an amino group, etc. 4. Identify the cofactor or other substrates that may be needed for that step. Oxidations require NAD+, reductions often require NADPH, amino additions require an –NH2 donor, p ...
INBORN ERRORS OF AMINO ACIDS METABOLISM
... When the body cannot break down tyrosine, high levels build up in the blood and form a toxic substance (known as succinylacetone) in the liver, kidneys, and central nervous system. This means that if tyrosinemia isn't treated, it may cause liver and kidney damage and brain-related problems, such as ...
... When the body cannot break down tyrosine, high levels build up in the blood and form a toxic substance (known as succinylacetone) in the liver, kidneys, and central nervous system. This means that if tyrosinemia isn't treated, it may cause liver and kidney damage and brain-related problems, such as ...
Cellular Respiration:
... tend to find these anaerobic organisms in extreme environments that might represent environments similar those of the early earth (before free oxygen was present). For example, in deep ocean hydrothermal vents we find heat-tolerant microbes that are killed by oxygen. We also find glycolysis in these ...
... tend to find these anaerobic organisms in extreme environments that might represent environments similar those of the early earth (before free oxygen was present). For example, in deep ocean hydrothermal vents we find heat-tolerant microbes that are killed by oxygen. We also find glycolysis in these ...
2 H
... • Cytochrome oxidase catalyzes the reduction of a final electron acceptor, oxygen • An artifcial e- donor, phenylenediamine, is used to reduce the cytochrome oxidase • If the enzyme is present, the colorless reagent (reduced state) will turn blue (oxidized state) ...
... • Cytochrome oxidase catalyzes the reduction of a final electron acceptor, oxygen • An artifcial e- donor, phenylenediamine, is used to reduce the cytochrome oxidase • If the enzyme is present, the colorless reagent (reduced state) will turn blue (oxidized state) ...
Document
... Endoplasmic (inside the cell); reticulum a network ER, a network inside the cell Disulfide bond formation occurs in the ER N-linked oligosaccharide synthesis is initiated in the ER; trimming and completion occurs in the Golgi Most O-glycosylation occurs in the Golgi Attachment of mannose 6-phosphate ...
... Endoplasmic (inside the cell); reticulum a network ER, a network inside the cell Disulfide bond formation occurs in the ER N-linked oligosaccharide synthesis is initiated in the ER; trimming and completion occurs in the Golgi Most O-glycosylation occurs in the Golgi Attachment of mannose 6-phosphate ...
1. Substrate level phosphorylation A) is part
... A) changes the muscles to a more type II fiber character B) changes muscles to a more type I fiber character C) makes mice more prone to obesity because they use more lipid as fuel D) both B and C ...
... A) changes the muscles to a more type II fiber character B) changes muscles to a more type I fiber character C) makes mice more prone to obesity because they use more lipid as fuel D) both B and C ...
SINGLE MOLECULE MAGNET Mn5-CYANIDE-
... In the limit of strong positive trigonal field the the orbital doublet 3 E ( ml 1 ) separated by the gap from the singlet 3 A2 is much higher in energy, the SO splitting (and local anisotropy) is fully suppressed, so the ground state is the orbital singlet 3 A2 which comprises m j 0 ml 0, ...
... In the limit of strong positive trigonal field the the orbital doublet 3 E ( ml 1 ) separated by the gap from the singlet 3 A2 is much higher in energy, the SO splitting (and local anisotropy) is fully suppressed, so the ground state is the orbital singlet 3 A2 which comprises m j 0 ml 0, ...
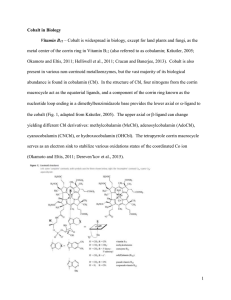
Cobalt Biology Discussion - 1-29-15
... metabolism. Acylation of activated olefin carbon double bonds catalyzed by B12 involving photoinduced cleavage of acetylcobalamin has also been performed (Walder and Orlinski, 1987), again demonstrating that cobalamin can be involved in light-activated abiological carbon fixation processes. ...
... metabolism. Acylation of activated olefin carbon double bonds catalyzed by B12 involving photoinduced cleavage of acetylcobalamin has also been performed (Walder and Orlinski, 1987), again demonstrating that cobalamin can be involved in light-activated abiological carbon fixation processes. ...
Oxy-haemoglobin protein engineering
... The oxy-haemoglobin protein is an important metalloprotein, which play a vital role in transport of oxygen [1-3]. It is well known that each subunit of haemoglobin (Hb) contains globular protein along with heme group. The protein tetramer comprises of two α- and two β-chains assembled to form symmet ...
... The oxy-haemoglobin protein is an important metalloprotein, which play a vital role in transport of oxygen [1-3]. It is well known that each subunit of haemoglobin (Hb) contains globular protein along with heme group. The protein tetramer comprises of two α- and two β-chains assembled to form symmet ...
Prelab Discussion
... Materials needed per team of two students: The 3 incubated SIM tubes from the last session. ...
... Materials needed per team of two students: The 3 incubated SIM tubes from the last session. ...
Document
... RNA similar to DNA except ◦ Contains ribose instead of deoxyribose ◦ Contains uracil instead of thymine ...
... RNA similar to DNA except ◦ Contains ribose instead of deoxyribose ◦ Contains uracil instead of thymine ...
Changes of cellular redox homeostasis and protein - LINK
... in many other events of the cellular life, such as signal transduction or protein degradation. A subset of chaperones takes part in the the formation of disulfide bonds, ion pairs and in the prolin cis-trans isomerisation of folding proteins. Disulfide bond formation is necessary for the active, fun ...
... in many other events of the cellular life, such as signal transduction or protein degradation. A subset of chaperones takes part in the the formation of disulfide bonds, ion pairs and in the prolin cis-trans isomerisation of folding proteins. Disulfide bond formation is necessary for the active, fun ...
factors_effecting_en..
... fastest. For most enzymes this is about pH 7-8 (physiological pH of most cells), but a few enzymes can work at extreme pH, such as protease enzymes in animal stomachs, which have an optimum of pH 1 The pH affects the charge of the amino acids at the active site, so the properties of the active site ...
... fastest. For most enzymes this is about pH 7-8 (physiological pH of most cells), but a few enzymes can work at extreme pH, such as protease enzymes in animal stomachs, which have an optimum of pH 1 The pH affects the charge of the amino acids at the active site, so the properties of the active site ...
crevier_osmium_1998
... shorter than those in 2, although it is presumably slightly longer than in 1. The data are most consistent with 1 acting as a p acid ligand in 2 and 3, as opposed to simply a dative (A) or s only (B) ligand. The CpCo(i) fragment forms two legged piano stool structures only with soft and/or p-acid li ...
... shorter than those in 2, although it is presumably slightly longer than in 1. The data are most consistent with 1 acting as a p acid ligand in 2 and 3, as opposed to simply a dative (A) or s only (B) ligand. The CpCo(i) fragment forms two legged piano stool structures only with soft and/or p-acid li ...
Whey Protein Concentrate
... Putting It All Together What does all this knowledge mean to you and how can you apply it to choose your protein wisely? Casein is digested slowly and releases amino acids into the blood gradually, with levels elevated even three hours later. Researchers found that casein did not effect protein synt ...
... Putting It All Together What does all this knowledge mean to you and how can you apply it to choose your protein wisely? Casein is digested slowly and releases amino acids into the blood gradually, with levels elevated even three hours later. Researchers found that casein did not effect protein synt ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.