90947 Investigate selected chemical reactions
... carbonates of copper, iron(II), zinc, calcium, and magnesium. Decomposition reactions. These are limited to thermal decomposition of carbonates and hydrogen carbonates. Displacement reactions. These are limited to the displacement of metal ions in solution by other metals. ...
... carbonates of copper, iron(II), zinc, calcium, and magnesium. Decomposition reactions. These are limited to thermal decomposition of carbonates and hydrogen carbonates. Displacement reactions. These are limited to the displacement of metal ions in solution by other metals. ...
Powerpoint
... The Protein Kinase-Like Superfamily • A range of different families, all phosphotransferases ...
... The Protein Kinase-Like Superfamily • A range of different families, all phosphotransferases ...
Document
... to convert NH4+ to urea, a less toxic molecule. Note that citrulline is transported across the inner membrane by a carrier for neutral amino acids. Ornithine is transported in exchange for H+ or citrulline. Fumarate is transported back into the mitochondrial matrix. Because the urea cycle was discov ...
... to convert NH4+ to urea, a less toxic molecule. Note that citrulline is transported across the inner membrane by a carrier for neutral amino acids. Ornithine is transported in exchange for H+ or citrulline. Fumarate is transported back into the mitochondrial matrix. Because the urea cycle was discov ...
Protein folding
... publicly available) are made available to a community that numbers more than 150 research groups around the world. ...
... publicly available) are made available to a community that numbers more than 150 research groups around the world. ...
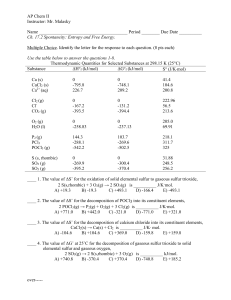
AP Chem II Instructor: Mr. Malasky Name Period ______ Due Date
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
cAMP-Dependent Protein Kinase, Catalytic Subunit Product
... in intracellular cAMP levels regulate cellular responses by influencing interaction between the Regulatory (R) and Catalytic (C) Subunits of PKA (6). The PKA holoenzyme exists as an inactive tetrameric complex (R2C2), which consists of a regulatory dimer (R2) associated with two Catalytic Subunits. ...
... in intracellular cAMP levels regulate cellular responses by influencing interaction between the Regulatory (R) and Catalytic (C) Subunits of PKA (6). The PKA holoenzyme exists as an inactive tetrameric complex (R2C2), which consists of a regulatory dimer (R2) associated with two Catalytic Subunits. ...
Copy of Acids, bases, salts answer key
... Limitations of Arrhenius theory : Arhhenius’ theory became quite popular and was widely accepted yet it had the following limitations: This theory was applicable only to aqueous solutions. Substances like Ammonia (NH3) do not contain hydroxide (OH) ion, even then its aqueous solution acts as a ...
... Limitations of Arrhenius theory : Arhhenius’ theory became quite popular and was widely accepted yet it had the following limitations: This theory was applicable only to aqueous solutions. Substances like Ammonia (NH3) do not contain hydroxide (OH) ion, even then its aqueous solution acts as a ...
Evolutionary relationship and application of a superfamily of cyclic
... (16%), they were well matched to each other within an rms deviation of 2.7 Å.18 These mechanistic properties and structural information clearly demonstrate that hydantoinase and dihydroorotase, as typical cyclic amidohydrolase superfamily enzymes, are evolutionarily closely related to each other. Bi ...
... (16%), they were well matched to each other within an rms deviation of 2.7 Å.18 These mechanistic properties and structural information clearly demonstrate that hydantoinase and dihydroorotase, as typical cyclic amidohydrolase superfamily enzymes, are evolutionarily closely related to each other. Bi ...
Genetic Code
... this tRNA, and which amino acid also bound in the active site of that enzyme, then you would know which amino acid will be found on this tRNA. And then you'd know what amino acid would go into the polypeptide when the mRNA had the codon UGG, which is complementary to this tRNA's anticodon. To make t ...
... this tRNA, and which amino acid also bound in the active site of that enzyme, then you would know which amino acid will be found on this tRNA. And then you'd know what amino acid would go into the polypeptide when the mRNA had the codon UGG, which is complementary to this tRNA's anticodon. To make t ...
complexes of transition metals with uramildiacetic acid
... extended the determinations of stability constants to cover the series of alkali metals (Li+, Na+, K+), thallium(I) and alkaline-earth metals (Be 2 +, Mg 2 +, Ca'+, Sr 2 +, Ba 2 +). It has been shown that the complexes of the alkali metals are stabilized by favourable enthalpy changes and that altho ...
... extended the determinations of stability constants to cover the series of alkali metals (Li+, Na+, K+), thallium(I) and alkaline-earth metals (Be 2 +, Mg 2 +, Ca'+, Sr 2 +, Ba 2 +). It has been shown that the complexes of the alkali metals are stabilized by favourable enthalpy changes and that altho ...
File - Pedersen Science
... 11. Briefly summarize what happens during the process of glycolysis. ****For the LOVE OF SCIENCE and Everything Catalytic and Enzymatic**** Concept 9.3: The citric acid cycle complete the energy –yielding oxidation of organic molecules 12. Using figure 9.10, explain the conversion of pyruvate in the ...
... 11. Briefly summarize what happens during the process of glycolysis. ****For the LOVE OF SCIENCE and Everything Catalytic and Enzymatic**** Concept 9.3: The citric acid cycle complete the energy –yielding oxidation of organic molecules 12. Using figure 9.10, explain the conversion of pyruvate in the ...
Chemistry 1
... In the box on the left, sketch 10 atoms of aluminum and 6 molecules of oxygen. In the box on the right, sketch the contents after the reaction of the particles occurs according to the balanced equation. ...
... In the box on the left, sketch 10 atoms of aluminum and 6 molecules of oxygen. In the box on the right, sketch the contents after the reaction of the particles occurs according to the balanced equation. ...
Chemical Reactions
... precipitate and which should not by circling either PPT or NO PPT. (Hint: look at the solubility rules) 1) PPT OR NO PPT ____NaNO3(aq) + ____AgNO3(aq) _________________ + _________________ 2) PPT OR NO PPT ____NaNO3(aq) + ____NaCl(aq) _________________ + _________________ 3) PPT OR NO PPT ____Na ...
... precipitate and which should not by circling either PPT or NO PPT. (Hint: look at the solubility rules) 1) PPT OR NO PPT ____NaNO3(aq) + ____AgNO3(aq) _________________ + _________________ 2) PPT OR NO PPT ____NaNO3(aq) + ____NaCl(aq) _________________ + _________________ 3) PPT OR NO PPT ____Na ...
CHAPTER 1 Differentiate b/w Mendeleev`s periodic law and modern
... The alkali metals have low ionization energy values, so they can give the electrons to other species very easily. In other words, they can decrease the oxidation number of other species and can act as reducing agents. Why the alkaline earth metals are reducing agents, but less reducing than those of ...
... The alkali metals have low ionization energy values, so they can give the electrons to other species very easily. In other words, they can decrease the oxidation number of other species and can act as reducing agents. Why the alkaline earth metals are reducing agents, but less reducing than those of ...
Answers to Selected Questions and Problems
... Group VIIA, the halogen family, all occur as diatomic molecules. neon Group VIIIA (18), the noble gases electrons (a) group IA (1); (b) group IIA (2); (c) group VIIA (17); (d) group VIA (16) (a) Na+; (b) O2−; (c) S2−; (d) Cl−; (e) Br− All alkali metal elements (group 1 or IA excluding hydrogen) The ...
... Group VIIA, the halogen family, all occur as diatomic molecules. neon Group VIIIA (18), the noble gases electrons (a) group IA (1); (b) group IIA (2); (c) group VIIA (17); (d) group VIA (16) (a) Na+; (b) O2−; (c) S2−; (d) Cl−; (e) Br− All alkali metal elements (group 1 or IA excluding hydrogen) The ...
Report 2 to hand in
... e) If the malachite samples we used were pure Cu2(OH)2CO3 - what should the mass percentage of copper be in the sample? f) If the malachite samples we used were pure Cu2(OH)2CO3 - what should the mass percentage of carbonate be in the sample? ...
... e) If the malachite samples we used were pure Cu2(OH)2CO3 - what should the mass percentage of copper be in the sample? f) If the malachite samples we used were pure Cu2(OH)2CO3 - what should the mass percentage of carbonate be in the sample? ...
WJEC Biology / Human Biology BY4 Question
... Most nitrogen-fixing bacteria form symbiotic associations with leguminous plants, where they are provided with nutrients by the plant and protected from oxygen. Oxygen inhibits the enzyme (nitrogenase) required for nitrogen fixation. Azotobacter are free-living in soil and have the ability to fix at ...
... Most nitrogen-fixing bacteria form symbiotic associations with leguminous plants, where they are provided with nutrients by the plant and protected from oxygen. Oxygen inhibits the enzyme (nitrogenase) required for nitrogen fixation. Azotobacter are free-living in soil and have the ability to fix at ...
UNIT 3 – CELLULAR ENERGETICS Chapter 9
... Name the three stages of cellular respiration and state the region of the eukaryotic cell where each stage occurs. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. Explain why ATP is required for the preparatory steps of glycolysis. Identify where substrate-leve ...
... Name the three stages of cellular respiration and state the region of the eukaryotic cell where each stage occurs. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. Explain why ATP is required for the preparatory steps of glycolysis. Identify where substrate-leve ...
Wound Healing and the Importance of Nutrition
... both have the same effect on the blood and therefore may thin the blood too much. For example, the supplement ‘Arginaid Powder’ contains no vitamin K, but ‘Arginaid Extra’ does. ‘Arginaid’ can be used in diabetic patients, as the tetra pack is a low GI product, however, there may be better control o ...
... both have the same effect on the blood and therefore may thin the blood too much. For example, the supplement ‘Arginaid Powder’ contains no vitamin K, but ‘Arginaid Extra’ does. ‘Arginaid’ can be used in diabetic patients, as the tetra pack is a low GI product, however, there may be better control o ...
The astounding health benefits of Chlorella and Spirulina
... cells, allowing the body to signal the cancerous cells to stop dividing. Therefore, foods rich in beta carotene may not only be able to prevent but also reverse cancers. (Wolf 1992)' (Spirulina, Nature's Superfood). Research as demonstrated that beta-carotene can lower cholesterol, treat wounds and ...
... cells, allowing the body to signal the cancerous cells to stop dividing. Therefore, foods rich in beta carotene may not only be able to prevent but also reverse cancers. (Wolf 1992)' (Spirulina, Nature's Superfood). Research as demonstrated that beta-carotene can lower cholesterol, treat wounds and ...
Answer Set 2
... exergonic reaction by maintaining the [products]/[reactants] ratio below the equilibrium value. This conversion is usually attained by using the products in another coupled reaction as soon as they are formed. 4. Liver: -45.2 kJ/mol; muscle: -48.1 kJ/mol; brain: -48.5 kJ/mol. The free energy of hydr ...
... exergonic reaction by maintaining the [products]/[reactants] ratio below the equilibrium value. This conversion is usually attained by using the products in another coupled reaction as soon as they are formed. 4. Liver: -45.2 kJ/mol; muscle: -48.1 kJ/mol; brain: -48.5 kJ/mol. The free energy of hydr ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.