Lesson 4 halogenoalkanes
... possibility there is for structural isomerism. The presence of a halogen atom is shown by the appropriate prefix: fluoro-, chloro-, bromo-, or iodo-. If the molecule contains more than one halogen atom of the same type this is shown by the prefixes di-, tri-, tetra, etc. The position of the halogen ...
... possibility there is for structural isomerism. The presence of a halogen atom is shown by the appropriate prefix: fluoro-, chloro-, bromo-, or iodo-. If the molecule contains more than one halogen atom of the same type this is shown by the prefixes di-, tri-, tetra, etc. The position of the halogen ...
Chemistry 199 - Department of Chemistry | Oregon State University
... Addition is to make an addition to a molecule. An example is the addition of a small molecule (such as Br2) to an alkene. ...
... Addition is to make an addition to a molecule. An example is the addition of a small molecule (such as Br2) to an alkene. ...
Chapter 1 Structure and Bonding
... 1) Reduction reactions can be reversed to give the aldehydes or ketones 2) Oxidizing Reagent is Cr(VI) a) (Na2Cr2O7 or K2Cr2O7 or CrO3) and H2SO4 and H2O R' ...
... 1) Reduction reactions can be reversed to give the aldehydes or ketones 2) Oxidizing Reagent is Cr(VI) a) (Na2Cr2O7 or K2Cr2O7 or CrO3) and H2SO4 and H2O R' ...
organic quiz 2
... the following will be the product(s)? a) 1-chlorohexane only b) 2-chlorohexane only c) 3-chlorohexane only d) both (b) and (c) 18) DNA is a natural polymer composed of a) glucose monomers b) nucleotide monomers c) amino acid monomers d) cellulose monomers 19) The process in which large organic molec ...
... the following will be the product(s)? a) 1-chlorohexane only b) 2-chlorohexane only c) 3-chlorohexane only d) both (b) and (c) 18) DNA is a natural polymer composed of a) glucose monomers b) nucleotide monomers c) amino acid monomers d) cellulose monomers 19) The process in which large organic molec ...
In the preparation of the esters given in this experiment
... 22. Give the structure and names of five alkenes having the molecular formula C6H12 that produce hexane on catalytic hydrogenation. 23. In an unsymmetrical alkene, the boron atom adds predominantly to the least substituted carbon atom. For example, 2-methyl-2-butene gives the products indicated in y ...
... 22. Give the structure and names of five alkenes having the molecular formula C6H12 that produce hexane on catalytic hydrogenation. 23. In an unsymmetrical alkene, the boron atom adds predominantly to the least substituted carbon atom. For example, 2-methyl-2-butene gives the products indicated in y ...
Document
... Changes The products of a chemical reaction may often be predicted by applying known facts about common reaction types ...
... Changes The products of a chemical reaction may often be predicted by applying known facts about common reaction types ...
Constitutional isomers and stereoisomers
... 2) An alcohol A with the molecular formula C4H10O can exist as enantiomers (optical isomers). i) State the structural requirement for a molecule to be able to exist as enantiomers. ii) Describe a property of enantiomers that would enable them to be distinguished from each other. iii) Draw the struct ...
... 2) An alcohol A with the molecular formula C4H10O can exist as enantiomers (optical isomers). i) State the structural requirement for a molecule to be able to exist as enantiomers. ii) Describe a property of enantiomers that would enable them to be distinguished from each other. iii) Draw the struct ...
Chemical Synthesis (sat6)
... A1: MgO and H2 -> Mg and H2O; A2: C and O2 -> CO2; A3: CO2 and H2O -> H2CO3; A4: MgO and H2 and O2 and C; minimize obj: H2CO3; Write(’Yes, H2CO3 is produced’); Write(’No, H2CO3 is not produced’); ...
... A1: MgO and H2 -> Mg and H2O; A2: C and O2 -> CO2; A3: CO2 and H2O -> H2CO3; A4: MgO and H2 and O2 and C; minimize obj: H2CO3; Write(’Yes, H2CO3 is produced’); Write(’No, H2CO3 is not produced’); ...
Shu Kobayashi Professor, University of Tokyo
... Substitutions of Alcohols in Water” Seiji Shirakawa and Shu Kobayashi Org. Lett. 2007, 9, 311-314 ...
... Substitutions of Alcohols in Water” Seiji Shirakawa and Shu Kobayashi Org. Lett. 2007, 9, 311-314 ...
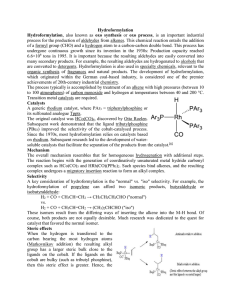
Hydroformylation Hydroformylation, also known as oxo synthesis or
... 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic s ...
... 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic s ...
Chemistry: Selected Topics
... some important types of chemical compounds. In the first part of the course, the general concepts of chemical reaction kinetics are presented with emphasis on the relation between reaction rate and reaction mechanism. The second part of the course deals with the properties of some important types of ...
... some important types of chemical compounds. In the first part of the course, the general concepts of chemical reaction kinetics are presented with emphasis on the relation between reaction rate and reaction mechanism. The second part of the course deals with the properties of some important types of ...
M_ScOrganic_Chemistr..
... Organic synthesis: Reterosynthsis, synthons and synthetic equivalents, functional group interconversion, Synthesis of amines, regiospecific, chemospecific and stereospecific reactions, umpolung methods. principles and applications of protective groups in protection of hydroxyl, amino, carbonyl and c ...
... Organic synthesis: Reterosynthsis, synthons and synthetic equivalents, functional group interconversion, Synthesis of amines, regiospecific, chemospecific and stereospecific reactions, umpolung methods. principles and applications of protective groups in protection of hydroxyl, amino, carbonyl and c ...
CHAPTER II. A Facile Synthesis of Arylacetic Acid Derivatives via
... “Design and Synthesis of Some Biologically Active Aryl Alkanoic Acid Derivatives and Development of Novel Synthetic Methodologies” The thesis has been presented as five chapters. Chapter-I deals with general introduction on the synthesis of arylacetic acids, -hydroxy arylacetic acid (mandelic acid) ...
... “Design and Synthesis of Some Biologically Active Aryl Alkanoic Acid Derivatives and Development of Novel Synthetic Methodologies” The thesis has been presented as five chapters. Chapter-I deals with general introduction on the synthesis of arylacetic acids, -hydroxy arylacetic acid (mandelic acid) ...
CHE 312 Exam III Review Sheet - Saint Leo University Faculty
... Explain why an aromatic molecule like benzene reacts differently than the corresponding alkene (actually a –triene)? ...
... Explain why an aromatic molecule like benzene reacts differently than the corresponding alkene (actually a –triene)? ...
Synthesis of Four Diastereomeric 3,5-Dialkoxy-2,4
... or, more recently, chiral catalysts, to produce the desired enantiomer with, at times, quite high selectivity. Several years ago we reported an alternative method for the preparation of all four diastereomeric aldol productss3-alkoxy-2methylalkanalsswith high enantiocontrol by a unique nonaldol rout ...
... or, more recently, chiral catalysts, to produce the desired enantiomer with, at times, quite high selectivity. Several years ago we reported an alternative method for the preparation of all four diastereomeric aldol productss3-alkoxy-2methylalkanalsswith high enantiocontrol by a unique nonaldol rout ...
Chapter 4 Reading Guide File
... 2. Differentiate between vitalism and mechanism. __________________________________________________ _________________________________________________________________________________________ 3. Carbon is said to be “tetravalent.” What does that mean? __________________________________________ 4. Exam ...
... 2. Differentiate between vitalism and mechanism. __________________________________________________ _________________________________________________________________________________________ 3. Carbon is said to be “tetravalent.” What does that mean? __________________________________________ 4. Exam ...
Chapter 1 Organoaluminum Reagents for Selective Organic
... Chapter 3. Bulky Aluminum Reagents for Selective Organic Synthesis In chapter 2 we discussed several excellent methods of discriminating various functional groups using bulky aluminum reagents. In this section we focus on the reactions promoted with bulky aluminum reagents which could not be achiev ...
... Chapter 3. Bulky Aluminum Reagents for Selective Organic Synthesis In chapter 2 we discussed several excellent methods of discriminating various functional groups using bulky aluminum reagents. In this section we focus on the reactions promoted with bulky aluminum reagents which could not be achiev ...
Lecture5
... -Reactive and relatively weak M-C bond -Absence of tendency to form M-M bond (in contrast to Ge, Sn) -Large variety of accessible coordination numbers and oxidation states - Application of 207Pb-NMR for the structure characterization ...
... -Reactive and relatively weak M-C bond -Absence of tendency to form M-M bond (in contrast to Ge, Sn) -Large variety of accessible coordination numbers and oxidation states - Application of 207Pb-NMR for the structure characterization ...
Perspective and prospects for pincer ligand chemistry
... with these metals. Variation of the metal, arm linkage, and phosphine alkyl groups led to the development of catalysts that produced alkene (+ dihydrogen) from alkane in concentrations approaching 0.5 M upon reflux in open systems! While terminal olefins are the kinetic products in these dehydrogena ...
... with these metals. Variation of the metal, arm linkage, and phosphine alkyl groups led to the development of catalysts that produced alkene (+ dihydrogen) from alkane in concentrations approaching 0.5 M upon reflux in open systems! While terminal olefins are the kinetic products in these dehydrogena ...
sridevi gopishetty - OASIS
... Worked for eight months (2002), as project assistant (isolation of natural product) at Regional Research Laboratory (CSIR), Jorhat, Assam, India Worked as a chemist for three months (2002), in Dr. Reddy’s Laboratory, one of the leading pharmaceutical industry in India Experience in design and synthe ...
... Worked for eight months (2002), as project assistant (isolation of natural product) at Regional Research Laboratory (CSIR), Jorhat, Assam, India Worked as a chemist for three months (2002), in Dr. Reddy’s Laboratory, one of the leading pharmaceutical industry in India Experience in design and synthe ...
Enantioselective synthesis

Enantioselective synthesis, also called chiral synthesis or asymmetric synthesis, is defined by IUPAC as: a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric (enantiomeric or diastereoisomeric) products in unequal amounts.Put more simply: it is the synthesis of a compound by a method that favors the formation of a specific enantiomer or diastereomer.Enantioselective synthesis is a key process in modern chemistry and is particularly important in the field of pharmaceuticals, as the different enantiomers or diastereomers of a molecule often have different biological activity.