chemistry - Kanpur University
... Alkenes, Cycloalkenes, Dienes and Alkynes: Nomenclature of alkenes, methods of formation, mechanisms of dehydration of alcohols and dehydrohalogenation of alkyl halids, regioselectivity in alcohol dehydration, The Saytzeff rule, Hofmann elimination, physical properties and relative stabilities of al ...
... Alkenes, Cycloalkenes, Dienes and Alkynes: Nomenclature of alkenes, methods of formation, mechanisms of dehydration of alcohols and dehydrohalogenation of alkyl halids, regioselectivity in alcohol dehydration, The Saytzeff rule, Hofmann elimination, physical properties and relative stabilities of al ...
Chemistry
... Learning chemistry requires both the assimilation of many concepts and the development of analytical skills. In this text, we have provided you with numerous tools to help you succeed in both tasks. If you are going to succeed in your chemistry course, you will have to develop good study habits. Sci ...
... Learning chemistry requires both the assimilation of many concepts and the development of analytical skills. In this text, we have provided you with numerous tools to help you succeed in both tasks. If you are going to succeed in your chemistry course, you will have to develop good study habits. Sci ...
Chemistry - Nagpur University
... potential, Reducing properties. Diagonal Relationships (Li-Mg) . Hydrogen bonding .Classification and effect of Hydrogen bonding on viscosity, solubility , M.pt. and B.pt (B)Chemistry of Noble Gases: Chemical properties of the noble gases, Chemistry of Xenon, Structure and bonding in xenon fluorides ...
... potential, Reducing properties. Diagonal Relationships (Li-Mg) . Hydrogen bonding .Classification and effect of Hydrogen bonding on viscosity, solubility , M.pt. and B.pt (B)Chemistry of Noble Gases: Chemical properties of the noble gases, Chemistry of Xenon, Structure and bonding in xenon fluorides ...
_______1. solution a. capable of being dissolved _______2. solute
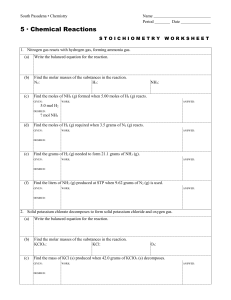
... 118. List three ways to increase the rate at which a reactions proceeds: _________________________________________________ _________________________________________________ _________________________________________________ 119. If a reaction takes place very slowly, the bonds that are broken and ref ...
... 118. List three ways to increase the rate at which a reactions proceeds: _________________________________________________ _________________________________________________ _________________________________________________ 119. If a reaction takes place very slowly, the bonds that are broken and ref ...
AP Chemistry
... system. Thus, the enthalpy change of a process is the same whether the process is carried out in one step or in a series of steps. Hess's law states that if a reaction is carried out in a series of steps, H for the reaction will be equal to the sum of the enthalpy changes for the steps. We can ther ...
... system. Thus, the enthalpy change of a process is the same whether the process is carried out in one step or in a series of steps. Hess's law states that if a reaction is carried out in a series of steps, H for the reaction will be equal to the sum of the enthalpy changes for the steps. We can ther ...
chemical reactions and energy changes
... Substances that are both strong electrolytes and acids are called strong acids. However, not all acids and bases are strong electrolytes. Look at Figure 3 (overleaf), which shows the conductivity of (a) pure water, (b) 0.2 m01 l-' HCl and (c) 0.2 m01 l-l acetic acid, as indicated by the lighting of ...
... Substances that are both strong electrolytes and acids are called strong acids. However, not all acids and bases are strong electrolytes. Look at Figure 3 (overleaf), which shows the conductivity of (a) pure water, (b) 0.2 m01 l-' HCl and (c) 0.2 m01 l-l acetic acid, as indicated by the lighting of ...
Matter and Measurement
... Electrolytes are characterized as being strong or weak. The strength of an electrolyte depends on the degree to which the compound dissociates in water to form ions. Hence ionic compounds like NaCl and K2SO4 which dissociate completely in water are strong electrolytes. Weak electrolytes do not diss ...
... Electrolytes are characterized as being strong or weak. The strength of an electrolyte depends on the degree to which the compound dissociates in water to form ions. Hence ionic compounds like NaCl and K2SO4 which dissociate completely in water are strong electrolytes. Weak electrolytes do not diss ...
Chemistry - Pearson School
... Learning chemistry requires both the assimilation of many concepts and the development of analytical skills. In this text, we have provided you with numerous tools to help you succeed in both tasks. If you are going to succeed in your chemistry course, you will have to develop good study habits. Sci ...
... Learning chemistry requires both the assimilation of many concepts and the development of analytical skills. In this text, we have provided you with numerous tools to help you succeed in both tasks. If you are going to succeed in your chemistry course, you will have to develop good study habits. Sci ...
Energy
... A coffee cup calorimeter contains 125. grams of water at 24.2oC. A 10.5 g sample of KBr, also at 24.2oC, is added. After dissolving, the mixture reaches a final temperature of 21.1oC. Calculate ∆Hsoln in joules/gram and kJ/mol. Assume the specific heat of the solution is 4.18 J/g-oC, and no heat is ...
... A coffee cup calorimeter contains 125. grams of water at 24.2oC. A 10.5 g sample of KBr, also at 24.2oC, is added. After dissolving, the mixture reaches a final temperature of 21.1oC. Calculate ∆Hsoln in joules/gram and kJ/mol. Assume the specific heat of the solution is 4.18 J/g-oC, and no heat is ...
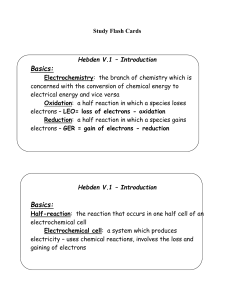
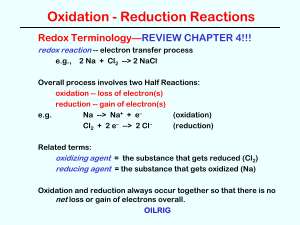
Hebden V.2 – Oxidation Numbers
... the halogens are usually –1 (Cl, Br, I, F) Polyatomic ions have an overall charge that will be shown like OHNeutral molecules do not have a charge shown – it is zero – H4P2O7 has a charge of 0 7. All atoms have charge of 0 8. Hydrogen in all compounds (except hydrides like LiH) is +1 – look to see i ...
... the halogens are usually –1 (Cl, Br, I, F) Polyatomic ions have an overall charge that will be shown like OHNeutral molecules do not have a charge shown – it is zero – H4P2O7 has a charge of 0 7. All atoms have charge of 0 8. Hydrogen in all compounds (except hydrides like LiH) is +1 – look to see i ...
Reduction
... In solutions in which [OH]= 1M, Mn(III) is stabilized with respect to reduction to Mn(II) as the value of Eo[OH]=1 for equation 8.35 illustrates. Compare this with ...
... In solutions in which [OH]= 1M, Mn(III) is stabilized with respect to reduction to Mn(II) as the value of Eo[OH]=1 for equation 8.35 illustrates. Compare this with ...
Equilibria PPT
... • A strong acid is one which is FULLY IONISED in water. It will have a high hydrogen ion concentration in this case ALL of the H+ is made so strong acid pH1 • A weak acid is one which is NOT fully ionised and is in equilibrium. It has a low hydrogen ion concentration In this case there are SOME H+ b ...
... • A strong acid is one which is FULLY IONISED in water. It will have a high hydrogen ion concentration in this case ALL of the H+ is made so strong acid pH1 • A weak acid is one which is NOT fully ionised and is in equilibrium. It has a low hydrogen ion concentration In this case there are SOME H+ b ...
1 Introduction
... Nearly a whole kilogram of waste for every kilogram of product! Remember, this is for the ideal case of 100% yield and 100% selectivity. In real life, the E-factor is usually much higher, because product yields are less than 100% and the reagents are often used in excess. Furthermore, in many cases ...
... Nearly a whole kilogram of waste for every kilogram of product! Remember, this is for the ideal case of 100% yield and 100% selectivity. In real life, the E-factor is usually much higher, because product yields are less than 100% and the reagents are often used in excess. Furthermore, in many cases ...
print
... Factors affecting reaction rates: 1) Nature of the reactants l Compounds with high energy bonds or elements with unstable electron configurations are prone to react (Their reactions have a lower activation energy, Ea). ...
... Factors affecting reaction rates: 1) Nature of the reactants l Compounds with high energy bonds or elements with unstable electron configurations are prone to react (Their reactions have a lower activation energy, Ea). ...
1 Intro / Review : Chemical Kinetics
... Also, the orientations of the reactant molecules during the collision must allow for the rearrangement of reactant bonds to form product bonds. Essential knowledge 4.B.3: A successful collision can be viewed as following a reaction path with an associated energy profile. Enduring understanding 4.D: ...
... Also, the orientations of the reactant molecules during the collision must allow for the rearrangement of reactant bonds to form product bonds. Essential knowledge 4.B.3: A successful collision can be viewed as following a reaction path with an associated energy profile. Enduring understanding 4.D: ...
Document
... IDEAL GAS LAW EXAMPLE Calculate pressure change in cylinder of a car’s engine when 0.250 g of octane [C8H18(l)] is burned with a stoichiometric amount of O2 assuming complete combustion. Cylinder volume = 0.100 L Initial Temp = 80 oC; final Temp = 700 oC ...
... IDEAL GAS LAW EXAMPLE Calculate pressure change in cylinder of a car’s engine when 0.250 g of octane [C8H18(l)] is burned with a stoichiometric amount of O2 assuming complete combustion. Cylinder volume = 0.100 L Initial Temp = 80 oC; final Temp = 700 oC ...
Enzyme catalysis

Enzyme catalysis is the increase in the rate of a chemical reaction by the active site of a protein. The protein catalyst (enzyme) may be part of a multi-subunit complex, and/or may transiently or permanently associate with a Cofactor (e.g. adenosine triphosphate). Catalysis of biochemical reactions in the cell is vital due to the very low reaction rates of the uncatalysed reactions. A key driver of protein evolution is the optimization of such catalytic activities via protein dynamics.The mechanism of enzyme catalysis is similar in principle to other types of chemical catalysis. By providing an alternative reaction route the enzyme reduces the energy required to reach the highest energy transition state of the reaction. The reduction of activation energy (Ea) increases the amount of reactant molecules that achieve a sufficient level of energy, such that they reach the activation energy and form the product. As with other catalysts, the enzyme is not consumed during the reaction (as a substrate is) but is recycled such that a single enzyme performs many rounds of catalysis.