H3AsO4 + 3 I- + 2 H3O+ H3AsO3 + I3- + H2O
... and decreases with distance between ions. Electronegativity measures the ability of an atom to attract electrons in a covalent bond. Electronegativity generally increases from left to right in the periodic table and decreases down a column. The difference in atoms' electronegativities is used to det ...
... and decreases with distance between ions. Electronegativity measures the ability of an atom to attract electrons in a covalent bond. Electronegativity generally increases from left to right in the periodic table and decreases down a column. The difference in atoms' electronegativities is used to det ...
The neutral theory of molecular evolution
... proposal may not seem particularly controversial now, it generated enormous controversy at the time, because at the time many paleoanthropologists interpreted the evidence to indicate humans diverged from apes as much as 30 million years ago. One year after Zuckerkandl and Pauling’s paper, Harris [ ...
... proposal may not seem particularly controversial now, it generated enormous controversy at the time, because at the time many paleoanthropologists interpreted the evidence to indicate humans diverged from apes as much as 30 million years ago. One year after Zuckerkandl and Pauling’s paper, Harris [ ...
FE Exam review for Chemistry
... C3H8 + 5O2 3CO2 + 4H2O reactants (change) products The carbon loses its hydrogen & hooks up with oxygen, creating CO2. Some of the oxygen combines with hydrogen to make water. Equations must be balanced. Same number & type of atoms on either side of the arrow. Good idea to save O & H for last. ...
... C3H8 + 5O2 3CO2 + 4H2O reactants (change) products The carbon loses its hydrogen & hooks up with oxygen, creating CO2. Some of the oxygen combines with hydrogen to make water. Equations must be balanced. Same number & type of atoms on either side of the arrow. Good idea to save O & H for last. ...
living environment
... answers for all multiple-choice questions, including those in Parts B–2 and D, on the separate answer sheet. Record your answers for all open-ended questions directly in this examination booklet. All answers in this examination booklet should be written in pen, except for graphs and drawings, which ...
... answers for all multiple-choice questions, including those in Parts B–2 and D, on the separate answer sheet. Record your answers for all open-ended questions directly in this examination booklet. All answers in this examination booklet should be written in pen, except for graphs and drawings, which ...
Transcript I
... Slide 5 - STEROID SYNTHETIC PATHWAYS General synthetic scheme to show you how these are manufactured. They all begin with cholesterol and go through several different pathways. Will end up with either a corticosterone, cortisol or an aldosterone. Corticosterone really isn’t a final product. ...
... Slide 5 - STEROID SYNTHETIC PATHWAYS General synthetic scheme to show you how these are manufactured. They all begin with cholesterol and go through several different pathways. Will end up with either a corticosterone, cortisol or an aldosterone. Corticosterone really isn’t a final product. ...
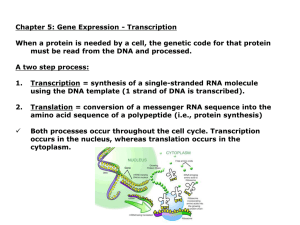
Gene expression: Transcription
... *S values of rRNAs refer to molecular size, as determined by sucrose gradient centrifugation. RNAs with larger S values are larger/have a greater density. ...
... *S values of rRNAs refer to molecular size, as determined by sucrose gradient centrifugation. RNAs with larger S values are larger/have a greater density. ...
Free energy
... The Regeneration of ATP • ATP is a renewable resource that is regenerated by addition of a phosphate group to adenosine diphosphate (ADP) • The energy to phosphorylate ADP comes from catabolic reactions in the cell • The ATP cycle is a revolving door through which energy passes during its transfer ...
... The Regeneration of ATP • ATP is a renewable resource that is regenerated by addition of a phosphate group to adenosine diphosphate (ADP) • The energy to phosphorylate ADP comes from catabolic reactions in the cell • The ATP cycle is a revolving door through which energy passes during its transfer ...
October 1 b. 1867 Wilder D. Bancroft, first systematic
... • John Dalton presented the first experimental evidence for atoms before the Manchester Literary & Philosophical Society, 1803. ...
... • John Dalton presented the first experimental evidence for atoms before the Manchester Literary & Philosophical Society, 1803. ...
Chem 12 UNIT TWO CHEMICAL EQUILIBRIUM 7.1 REVERSIBLE
... ENTROPY TO CHEMICAL EQUILIBRIUM: These two tendencies decide where the balance will be struck between PRODUCTS and REACTANTS during a chemical or physical change. Entropy and energy (ENTHALPY) determine which side of the reaction wins and how much it wins by... eg. 20% reactants and 80% products at ...
... ENTROPY TO CHEMICAL EQUILIBRIUM: These two tendencies decide where the balance will be struck between PRODUCTS and REACTANTS during a chemical or physical change. Entropy and energy (ENTHALPY) determine which side of the reaction wins and how much it wins by... eg. 20% reactants and 80% products at ...
Chemical Composition
... • If I want to know how many O2 molecules I will need or how many CO2 molecules I can make, I will need to know how many C atoms are in the sample of carbon I am starting with • Dalton used the percentages of elements in compounds and the chemical formulas to deduce the relative masses of atoms • Un ...
... • If I want to know how many O2 molecules I will need or how many CO2 molecules I can make, I will need to know how many C atoms are in the sample of carbon I am starting with • Dalton used the percentages of elements in compounds and the chemical formulas to deduce the relative masses of atoms • Un ...
Pulmonary System_Lecture II - Medical
... Respiration is achieved through the mouth, nose, trachea, lungs, and diaphragm. Oxygen enters the respiratory system through the mouth and the nose. The oxygen then passes through the larynx (where speech sounds are produced) and the trachea which is a tube that enters the chest cavity. In the chest ...
... Respiration is achieved through the mouth, nose, trachea, lungs, and diaphragm. Oxygen enters the respiratory system through the mouth and the nose. The oxygen then passes through the larynx (where speech sounds are produced) and the trachea which is a tube that enters the chest cavity. In the chest ...
Seminario Glúcidos 3 y lípidos 1. Comente los mecanismos de
... mitochondria free of extraneous elements (6). We have found that these preparations are contaminated to a small degree with erythrocytes. These extraneous elements may be removed by taking up the unwashed pellet of mitochondria which had been sedimented once in 10 volumes of 0.88 M sucrose as descri ...
... mitochondria free of extraneous elements (6). We have found that these preparations are contaminated to a small degree with erythrocytes. These extraneous elements may be removed by taking up the unwashed pellet of mitochondria which had been sedimented once in 10 volumes of 0.88 M sucrose as descri ...
Lipid Oxidation
... radical may shift to carbon 14 with the double bond reforming between carbons 11 and 12. The radical may also shift to carbon 9 with the double bond forming between carbons 10 and 11. Both of these cases result in conjugated structures that are at lower energies than are the non conjugated structure ...
... radical may shift to carbon 14 with the double bond reforming between carbons 11 and 12. The radical may also shift to carbon 9 with the double bond forming between carbons 10 and 11. Both of these cases result in conjugated structures that are at lower energies than are the non conjugated structure ...
presentation source
... transcriptional regulation of gene expression leading to defects in metabolic signaling of insulin secretion, beta cell mass or both Usual treatment (?) ...
... transcriptional regulation of gene expression leading to defects in metabolic signaling of insulin secretion, beta cell mass or both Usual treatment (?) ...
North Carolina Test of Biology
... © 2009 All rights reserved. This document may not be reproduced by any means, in whole or in part, without prior written permission from the North Carolina Department of Public Instruction, Raleigh, North Carolina. ...
... © 2009 All rights reserved. This document may not be reproduced by any means, in whole or in part, without prior written permission from the North Carolina Department of Public Instruction, Raleigh, North Carolina. ...
Exam Review 1: CHM 1411 Time: 0hr 55mins
... B) neutrons in nucleus; protons and electrons in orbitals C) protons and neutrons in nucleus; electrons in orbitals D) protons and electrons in nucleus; neutrons in orbitals E) electrons in nucleus; protons and neutrons in orbitals Answer: C 14) The mass number is equal to A) the sum of the number o ...
... B) neutrons in nucleus; protons and electrons in orbitals C) protons and neutrons in nucleus; electrons in orbitals D) protons and electrons in nucleus; neutrons in orbitals E) electrons in nucleus; protons and neutrons in orbitals Answer: C 14) The mass number is equal to A) the sum of the number o ...
Diapositiva 1 - Universidad Autónoma de San Luis Potosí
... • Slight variations on the canonical code had been predicted • Alternative codes were discovered in 1979, in human mitochondria. • Many alternative mitochondrial codes now known. • Mycoplasma variants translate UGA as tryptophan. ...
... • Slight variations on the canonical code had been predicted • Alternative codes were discovered in 1979, in human mitochondria. • Many alternative mitochondrial codes now known. • Mycoplasma variants translate UGA as tryptophan. ...
Solution Preparation Final Goueth
... (D) The average velocity of the O2 molecules is one-half that of the CH4 molecules. 65. 168.00 J of energy are added to a sample of gallium initially at 25.0 °C, the temperature rises to 38.0 °C. What is the volume of the sample? Data for Gallium, Ga specific heat 0.372 J g¯1 °C¯1 ...
... (D) The average velocity of the O2 molecules is one-half that of the CH4 molecules. 65. 168.00 J of energy are added to a sample of gallium initially at 25.0 °C, the temperature rises to 38.0 °C. What is the volume of the sample? Data for Gallium, Ga specific heat 0.372 J g¯1 °C¯1 ...
Biology Priority Expectations
... Describe the composition of the four major categories of organic molecules (carbohydrates, lipids, proteins, and nucleic acids). ...
... Describe the composition of the four major categories of organic molecules (carbohydrates, lipids, proteins, and nucleic acids). ...
03_Membrane rest potential. Generation and radiation action
... and plasma membranes, catalyze ATP-dependent transport of Ca++ away from the cytosol, into the ER lumen or out of the cell. Some evidence indicates that these pumps may be antiporters, transporting protons in the opposite direction. Ca++-ATPase pumps function to keep cytosolic Ca++ low, allowing Ca+ ...
... and plasma membranes, catalyze ATP-dependent transport of Ca++ away from the cytosol, into the ER lumen or out of the cell. Some evidence indicates that these pumps may be antiporters, transporting protons in the opposite direction. Ca++-ATPase pumps function to keep cytosolic Ca++ low, allowing Ca+ ...
Sustained nonoxidative glucose utilization and depletion of
... tion dogs received a priming bolus of 20 &i of L-[1-‘4C]lactic acid (New England Nuclear Corp.; specific activity 55 mCi/mmol) followed by constant intravenous infusion at a rate of 25 &i/h. After an equilibration period of 25 min (16), arterial and venous samples were withdrawn as outlined to deter ...
... tion dogs received a priming bolus of 20 &i of L-[1-‘4C]lactic acid (New England Nuclear Corp.; specific activity 55 mCi/mmol) followed by constant intravenous infusion at a rate of 25 &i/h. After an equilibration period of 25 min (16), arterial and venous samples were withdrawn as outlined to deter ...
Understanding Our Environment
... Use of fossil fuels, deforestation, and other human activities have added excess carbon dioxide to the atmosphere. - May enhance photosynthesis. Plants may counter-balance by developing fewer stomata. Stern - Introductory Plant Biology: 9th Ed. - All Rights Reserved - McGraw Hill Companies ...
... Use of fossil fuels, deforestation, and other human activities have added excess carbon dioxide to the atmosphere. - May enhance photosynthesis. Plants may counter-balance by developing fewer stomata. Stern - Introductory Plant Biology: 9th Ed. - All Rights Reserved - McGraw Hill Companies ...
a
... Sodium and chloride help maintain normal osmolarity, water balance, and are essential in nerve and muscle function ...
... Sodium and chloride help maintain normal osmolarity, water balance, and are essential in nerve and muscle function ...
Biochemistry
_and_Carl_Ferdinand_Cori.jpg?width=300)
Biochemistry, sometimes called biological chemistry, is the study of chemical processes within and relating to living organisms. By controlling information flow through biochemical signaling and the flow of chemical energy through metabolism, biochemical processes give rise to the complexity of life. Over the last decades of the 20th century, biochemistry has become so successful at explaining living processes that now almost all areas of the life sciences from botany to medicine to genetics are engaged in biochemical research. Today, the main focus of pure biochemistry is in understanding how biological molecules give rise to the processes that occur within living cells, which in turn relates greatly to the study and understanding of whole organisms.Biochemistry is closely related to molecular biology, the study of the molecular mechanisms by which genetic information encoded in DNA is able to result in the processes of life. Depending on the exact definition of the terms used, molecular biology can be thought of as a branch of biochemistry, or biochemistry as a tool with which to investigate and study molecular biology.Much of biochemistry deals with the structures, functions and interactions of biological macromolecules, such as proteins, nucleic acids, carbohydrates and lipids, which provide the structure of cells and perform many of the functions associated with life. The chemistry of the cell also depends on the reactions of smaller molecules and ions. These can be inorganic, for example water and metal ions, or organic, for example the amino acids which are used to synthesize proteins. The mechanisms by which cells harness energy from their environment via chemical reactions are known as metabolism. The findings of biochemistry are applied primarily in medicine, nutrition, and agriculture. In medicine, biochemists investigate the causes and cures of disease. In nutrition, they study how to maintain health and study the effects of nutritional deficiencies. In agriculture, biochemists investigate soil and fertilizers, and try to discover ways to improve crop cultivation, crop storage and pest control.