Electrons in Atoms
... • if photons are waves behaving like particles, then perhaps particles can have some of the properties of waves B. The Apparent Contradiction • Are e- waves or particles? BOTH!!, the wave-particle duality of nature allows one to focus on the properties most important for their studies. ...
... • if photons are waves behaving like particles, then perhaps particles can have some of the properties of waves B. The Apparent Contradiction • Are e- waves or particles? BOTH!!, the wave-particle duality of nature allows one to focus on the properties most important for their studies. ...
density functional theory
... This thesis provides an introduction to basic, wave-function based many-body quantum mechanics as well as to the theoretical foundations of DFT up to the point where iteratively accessible, density-based equations for both, time-independent and timedependent problems are presented. A major component ...
... This thesis provides an introduction to basic, wave-function based many-body quantum mechanics as well as to the theoretical foundations of DFT up to the point where iteratively accessible, density-based equations for both, time-independent and timedependent problems are presented. A major component ...
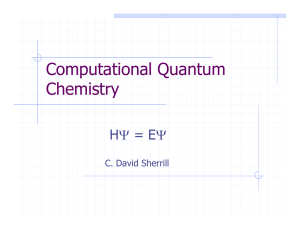
Computational Quantum Chemistry
... for the mathematical theory of a large part of physics and the whole of chemistry are thus completely known and the difficulty is only that the exact application of these laws leads to equations much too complicated to be soluble.” ---P.A.M. Dirac, Proc. Roy. Soc. (London), 123 714 (1929). ...
... for the mathematical theory of a large part of physics and the whole of chemistry are thus completely known and the difficulty is only that the exact application of these laws leads to equations much too complicated to be soluble.” ---P.A.M. Dirac, Proc. Roy. Soc. (London), 123 714 (1929). ...
Pauli Exclusion Principle
... is that it does not tell us how the three 2p electrons are distributed among the three 2p orbitals. We can show this by using an orbital diagram in which boxes are used to indicate orbitals within subshells and arrows to represent electrons in these orbitals. The direction of the arrows represent th ...
... is that it does not tell us how the three 2p electrons are distributed among the three 2p orbitals. We can show this by using an orbital diagram in which boxes are used to indicate orbitals within subshells and arrows to represent electrons in these orbitals. The direction of the arrows represent th ...
Electron Configuration
... This involves looking at the most recent noble gas (since they are stable and all orbitals are full) and then continuing the electron configuration from there ...
... This involves looking at the most recent noble gas (since they are stable and all orbitals are full) and then continuing the electron configuration from there ...
Multi-Electron Atoms Helium Schrödinger Equation
... Gives electronic states for helium without electron-electron interaction (and without exchange and spin-orbit). Only order of magnitude agreement with experiment due to drastic approximation. Systematically overestimates the ...
... Gives electronic states for helium without electron-electron interaction (and without exchange and spin-orbit). Only order of magnitude agreement with experiment due to drastic approximation. Systematically overestimates the ...
the quantum mechanical potential for the prime numbers
... The existence of a potential which admits all the prime numbers as its only eigenvalues has some important implications. For instance, a long-standing issue in number theory such as the primality test of a given number N can be completely resolved by the sole application of physical laws. To do this ...
... The existence of a potential which admits all the prime numbers as its only eigenvalues has some important implications. For instance, a long-standing issue in number theory such as the primality test of a given number N can be completely resolved by the sole application of physical laws. To do this ...
Lecture 4 (October 1, 2007): Quantum Statistical Mechanics
... where Ze is the charge of the nucleus and 137.04 is the dimensionless “fine structure constant”. Since me c 2 0.511 MeV , the ground state energy (equal to the ionization energy) for hydrogen is EGH E1 13.6 eV ...
... where Ze is the charge of the nucleus and 137.04 is the dimensionless “fine structure constant”. Since me c 2 0.511 MeV , the ground state energy (equal to the ionization energy) for hydrogen is EGH E1 13.6 eV ...
Conductivities and transmission coefficients of ultra-thin disordered metallic films B. J.
... the film thickness [9]. We calculated the conductivity for discrete values of the thickness (corresponding to full monolayers), and the deviations from monotonous behaviour occur between the points and cannot be seen. Additionally, we find the relation between our model and a formalism based on the ...
... the film thickness [9]. We calculated the conductivity for discrete values of the thickness (corresponding to full monolayers), and the deviations from monotonous behaviour occur between the points and cannot be seen. Additionally, we find the relation between our model and a formalism based on the ...
8.044s13 Excited State Helium, He
... cloud produced by taking the charge distribution resulting from our wavefunctions. The greatest contributions would come from spots where the two electrons were closest together. In the triplet case the wavefunction goes to zero when the two electrons approach each other. ...
... cloud produced by taking the charge distribution resulting from our wavefunctions. The greatest contributions would come from spots where the two electrons were closest together. In the triplet case the wavefunction goes to zero when the two electrons approach each other. ...
Part IV
... edge, k = (π/a). For k near this value, we must use the exact L(ε/Vo) expression. • It can be shown (S, Ch. 2) that, in limit of small barriers (|Vo| << ε), the exact expression for the Krönig-Penney effective mass at the BZ edge is: ...
... edge, k = (π/a). For k near this value, we must use the exact L(ε/Vo) expression. • It can be shown (S, Ch. 2) that, in limit of small barriers (|Vo| << ε), the exact expression for the Krönig-Penney effective mass at the BZ edge is: ...
Electrons in a Shell - University of California, Berkeley
... numerical integration of the T-F equation [2]. Instead of this, in what follows we prove that for sufficiently large N, the electron density is concentrated in a thin layer of characteristic thickness δ near the wall (see Figure), and we give a rough estimate of δ, showing how this parameter scales ...
... numerical integration of the T-F equation [2]. Instead of this, in what follows we prove that for sufficiently large N, the electron density is concentrated in a thin layer of characteristic thickness δ near the wall (see Figure), and we give a rough estimate of δ, showing how this parameter scales ...
Pride, Prejudice, and Penury of ab initio transport calculations for
... Still, ground and excited states of correlated electron systems are not single Slater determinants and therefore the validity of applying scattering theories designed for non-interacting particles to interacting systems is not straightforward to establish. Moreover, including correlation effects bey ...
... Still, ground and excited states of correlated electron systems are not single Slater determinants and therefore the validity of applying scattering theories designed for non-interacting particles to interacting systems is not straightforward to establish. Moreover, including correlation effects bey ...
Condensed Plasmoids – The Intermediate State of LENR
... Based on the theory above the author is undertaking ab initio (i.e. derived only from first principles) quantummechanical simulations of CPs. This is the subject of ongoing research. The goal is to obtain the quantitative properties of CP, such as its densities, intrinsic current and bonding energy. ...
... Based on the theory above the author is undertaking ab initio (i.e. derived only from first principles) quantummechanical simulations of CPs. This is the subject of ongoing research. The goal is to obtain the quantitative properties of CP, such as its densities, intrinsic current and bonding energy. ...
White Dwarf Properties and the Degenerate Electron Gas
... where v(p) and ǫ(p) are the relativistic expressions for the velocity and energy of a given momentum state, respectively. µ has dimensions of energy and is called the chemical potential. In this context it is related to the degree to which the interior is degenerate. This formula cannot be solved an ...
... where v(p) and ǫ(p) are the relativistic expressions for the velocity and energy of a given momentum state, respectively. µ has dimensions of energy and is called the chemical potential. In this context it is related to the degree to which the interior is degenerate. This formula cannot be solved an ...
E618: Pertubation theory for Helium atom
... The stable states are those with the energies between Eground = −M α2 (1 + 1) andE∞ = −M α2 (1 + 0) as above E∞ , the atom can be ionized (one of the electrons is taken to infinity). all the states below E∞ are stable, and all the states above it belong to the continum. Thus, the stable states are t ...
... The stable states are those with the energies between Eground = −M α2 (1 + 1) andE∞ = −M α2 (1 + 0) as above E∞ , the atom can be ionized (one of the electrons is taken to infinity). all the states below E∞ are stable, and all the states above it belong to the continum. Thus, the stable states are t ...
Tutorial 3 - answers • Complete the following table, giving either the
... Hund’s rule states that when filling orbitals of the same energy, there is the maximum number of unpaired electron spins (or orbitals of the same energy are filled singularly first before electrons are paired up). ...
... Hund’s rule states that when filling orbitals of the same energy, there is the maximum number of unpaired electron spins (or orbitals of the same energy are filled singularly first before electrons are paired up). ...
Semiconductor Physics
... the Kronig-Penney model. At high doping the impurity band overlap with the conduction band. ...
... the Kronig-Penney model. At high doping the impurity band overlap with the conduction band. ...
Quantum mechanics
... – Instead, we imagine each electron interacting with a static field of all of the other electrons – According to the variational principle, the lowest energy will can get with HF theory will always be greater than the true energy of the system • The difference is the correlation energy ...
... – Instead, we imagine each electron interacting with a static field of all of the other electrons – According to the variational principle, the lowest energy will can get with HF theory will always be greater than the true energy of the system • The difference is the correlation energy ...
Quantum Chemistry and Spectroscopy
... Naturally the GGA methods have some problems. One of them is the van der Waals interactions (or dispersion) which is important in weakly bonded systems. Basically HF and GGA will do not have them and they have to be added using additional (empirical) terms. In most cases such PBE-D3 models works w ...
... Naturally the GGA methods have some problems. One of them is the van der Waals interactions (or dispersion) which is important in weakly bonded systems. Basically HF and GGA will do not have them and they have to be added using additional (empirical) terms. In most cases such PBE-D3 models works w ...
The influence of effective mass on magnetoresistance in ultrathin Fe/Cr/Fe films K. W
... these oscillations depends on the effective mass. In particular, when we compare the upper curve in Fig.1 (dotted line), obtained by means of the Hood–Falicov approach (m* = 2me), with the curve in Fig. 2 (solid line) for the same effective mass ( m ∗ = 2me ), we can see that the appearance of oscil ...
... these oscillations depends on the effective mass. In particular, when we compare the upper curve in Fig.1 (dotted line), obtained by means of the Hood–Falicov approach (m* = 2me), with the curve in Fig. 2 (solid line) for the same effective mass ( m ∗ = 2me ), we can see that the appearance of oscil ...
ELECTRON I: Free electron model
... Free electron gas: good approximation for metals The importance of Drude model is that it can be used to form quick, intuitive pictures for metals and heavily doped semiconductors. (We will talk about doping in great detail later in this course.) Its basic assumptions are discussed in the following. ...
... Free electron gas: good approximation for metals The importance of Drude model is that it can be used to form quick, intuitive pictures for metals and heavily doped semiconductors. (We will talk about doping in great detail later in this course.) Its basic assumptions are discussed in the following. ...