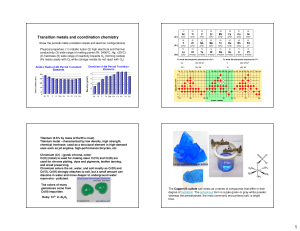
IJCA 39A(8) 792-801
... formaldehyde system , C=O bond length decreases in the metal-formaldehyde system. This may be due to charge transfer interaction between metal ion and oxygen lone pair electrons. The decreased electron density is compensated from the other atoms in the l8 molecule . So it is important to rationalize ...
... formaldehyde system , C=O bond length decreases in the metal-formaldehyde system. This may be due to charge transfer interaction between metal ion and oxygen lone pair electrons. The decreased electron density is compensated from the other atoms in the l8 molecule . So it is important to rationalize ...
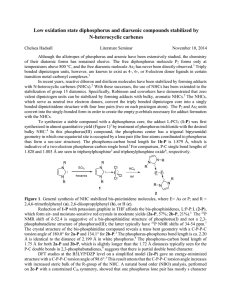
NOVEL IRON(II) TRIAZOLE-PYRIDINE BASED DINUCLEAR COMPLEXES: SYNTHESIS, CHARACTERIZATION AND MAGNETIC PROPERTIES
... steric hindrance in the complexes and decrease the ligand field strength. As a result, the influence of the methyl group may induce SCO in low spin (LS) complexes, or tune the SCO properties (such as transition hysteresis or transition temperature) in complexes with SCO. Ligands were divided into th ...
... steric hindrance in the complexes and decrease the ligand field strength. As a result, the influence of the methyl group may induce SCO in low spin (LS) complexes, or tune the SCO properties (such as transition hysteresis or transition temperature) in complexes with SCO. Ligands were divided into th ...
Sample 3 - University of Puget Sound
... The proposed research involves the multistep synthesis of organic and organometallic complexes by using techniques and strategies that are well known. However, the target compounds have not been previously synthesized using these strategies. As such, each individual step of the proposed syntheses wi ...
... The proposed research involves the multistep synthesis of organic and organometallic complexes by using techniques and strategies that are well known. However, the target compounds have not been previously synthesized using these strategies. As such, each individual step of the proposed syntheses wi ...
Ball Lab ChemDraw guide copy
... Figure 6: Drawing and ideal angles of B(OH)2 a trigonal complex. ...
... Figure 6: Drawing and ideal angles of B(OH)2 a trigonal complex. ...
Ligand effects on the X-ray absorption of a nickel
... of the XANES by 0.4 eV. All atoms out to 5.0 Angstroms were used in the matrix inversion of Eq. (14) to find the XANES. Using the X-ray diffraction geometry of the NiTPP(Pip)2 molecule, the XANES signatures of the ligands were investigated by selectively removing one or both ligands and repeating the ...
... of the XANES by 0.4 eV. All atoms out to 5.0 Angstroms were used in the matrix inversion of Eq. (14) to find the XANES. Using the X-ray diffraction geometry of the NiTPP(Pip)2 molecule, the XANES signatures of the ligands were investigated by selectively removing one or both ligands and repeating the ...
Project Title Here (in Bold 14 point Times font)
... V[TCNE] V TCNE TCNE combined organic/inorganic molecular systems to create locally accessible 2d spin networks. Here we V[TCNE]2 1nm TCNE have used tetracyanoethylene (TCNE) molecules combined with vanadium adatoms. TCNE is a flat, sp2-bonded molecule that has an extremely high step 1 step 2 step 3 ...
... V[TCNE] V TCNE TCNE combined organic/inorganic molecular systems to create locally accessible 2d spin networks. Here we V[TCNE]2 1nm TCNE have used tetracyanoethylene (TCNE) molecules combined with vanadium adatoms. TCNE is a flat, sp2-bonded molecule that has an extremely high step 1 step 2 step 3 ...
Chemistry (Theory)
... Variation of molar conductivities with dilution: In case of weak electrolyte: On increasing the dilution, undissociated weak electrolytes dissociate in small extent further so, the number of conducting ions increases resulting increase in the molar conductivity. In case of strong electrolyte: In the ...
... Variation of molar conductivities with dilution: In case of weak electrolyte: On increasing the dilution, undissociated weak electrolytes dissociate in small extent further so, the number of conducting ions increases resulting increase in the molar conductivity. In case of strong electrolyte: In the ...
Reactivity of Transition Metal Organometallics L. J. Farrugia MSc
... 1.3 Oxidation states in Cp compounds Since Cp is formally charged with a -ve charge, each Cp ligand present in a compound contributes a charge of +1 towards the formal oxidation state of the metal. Hence a metal must be in a +ve oxidation level. The only exception would be with +ve charged ligands - ...
... 1.3 Oxidation states in Cp compounds Since Cp is formally charged with a -ve charge, each Cp ligand present in a compound contributes a charge of +1 towards the formal oxidation state of the metal. Hence a metal must be in a +ve oxidation level. The only exception would be with +ve charged ligands - ...
option 1 - IIT Bombay
... two types: t2g and eg 3.Complex compounds: compounds made most commonly by Transition metals (like iron, copper, nickel) which involve special bonds, and hence these are classified seperately as complex””. 4.Ligands: These are negatively charged compounds which attach to a transition metal to make c ...
... two types: t2g and eg 3.Complex compounds: compounds made most commonly by Transition metals (like iron, copper, nickel) which involve special bonds, and hence these are classified seperately as complex””. 4.Ligands: These are negatively charged compounds which attach to a transition metal to make c ...
Spin crossover

Spin Crossover (SCO), sometimes referred to as spin transition or spin equilibrium behavior, is a phenomenon that occurs in some metal complexes wherein the spin state of the complex changes due to external stimuli such as a variation of temperature, pressure, light irradiation or an influence of a magnetic field.With regard to a ligand field and ligand field theory, the change in spin state is a transition from a low spin (LS) ground state electron configuration to a high spin (HS) ground state electron configuration of the metal’s d atomic orbitals (AOs), or vice versa. The magnitude of the ligand field splitting along with the pairing energy of the complex determines whether it will have a LS or HS electron configuration. A LS state occurs because the ligand field splitting (Δ) is greater than the pairing energy of the complex (which is an unfavorable process).Figure 1 is a simplified illustration of the metal’s d orbital splitting in the presence of an octahedral ligand field. A large splitting between the t2g and eg AOs requires a substantial amount of energy for the electrons to overcome the energy gap (Δ) to comply with Hund’s Rule. Therefore, electrons will fill the lower energy t2g orbitals completely before populating the higher energy eg orbitals. Conversely, a HS state occurs with weaker ligand fields and smaller orbital splitting. In this case the energy required to populate the higher levels is substantially less than the pairing energy and the electrons fill the orbitals according to Hund’s Rule by populating the higher energy orbitals before pairing with electrons in the lower lying orbitals. An example of a metal ion that can exist in either a LS or HS state is Fe3+ in an octahedral ligand field. Depending on the ligands that are coordinated to this complex the Fe3+ can attain a LS or a HS state, as in Figure 1.Spin crossover refers to the transitions between high to low, or low to high, spin states. This phenomenon is commonly observed with some first row transition metal complexes with a d4 through d7 electron configuration in an octahedral ligand geometry. Spin transition curves are a common representation of SCO phenomenon with the most commonly observed types depicted in Figure 2 in which γHS (the high-spin molar fraction) is plotted vs. T. The figure shows a gradual spin transition (left), an abrupt transition with hysteresis (middle) and a two-step transition (right). For a transition to be considered gradual, it typically takes place over a large temperature range, even up to several hundred K, whereas for a transition to be considered abrupt, it should take place within 10 K or less.These curves indicate that a spin transition has occurred in a metal complex as temperature changed. The gradual transition curve is an indication that not all metal centers within the complex are undergoing the transition at the same temperature. The abrupt spin change with hysteresis indicates a strong cooperativity, or “communication”, between neighboring metal complexes. In the latter case, the material is bistable and can exist in the two different spin states with a different range of external stimuli (temperature in this case) for the two phenomena, namely LS → HS and HS → LS. The two-step transition is relatively rare but is observed, for example, with dinuclear SCO complexes for which the spin transition in one metal center renders the transition in the second metal center less favorable.There are several types of spin crossover that can occur in a complex; some of them are light induced excited state spin trapping (LIESST), ligand-driven light induced spin change (LD-LISC), and charge transfer induced spin transition (CTIST).