reductive elimination
... After reductive elimination, a T-shaped 3-coordinate species is formed. If the above mechanism is correct, then oxidative addition at 3-coordinate RhCl(PR3)2 should also be facile. Indeed, RhCl(PPh3)2 formed by dissociation of one PPh3 from RhCl(PPh3)3 undergoes oxidative addition with H2 at a rate ...
... After reductive elimination, a T-shaped 3-coordinate species is formed. If the above mechanism is correct, then oxidative addition at 3-coordinate RhCl(PR3)2 should also be facile. Indeed, RhCl(PPh3)2 formed by dissociation of one PPh3 from RhCl(PPh3)3 undergoes oxidative addition with H2 at a rate ...
Experiment 8 Chelatometric Analysis of the Complex for Cobalt
... the final adjustment of the pH as indicated by a change to yellow by the metal-murexide complex (probably CoH2 D- now). Titrating the solution with standard EDTA first complexes the free cobalt ions and then finally complexes the cobalt which was up-to-now complexed by the murexide. The end point is ...
... the final adjustment of the pH as indicated by a change to yellow by the metal-murexide complex (probably CoH2 D- now). Titrating the solution with standard EDTA first complexes the free cobalt ions and then finally complexes the cobalt which was up-to-now complexed by the murexide. The end point is ...
Lecture 15 16 - TAMU Chemistry
... Classifications of Ligands 1. type of bonding they engage in π-donation, ...
... Classifications of Ligands 1. type of bonding they engage in π-donation, ...
Document
... • For this reason, a transition metal is defined as being an element which forms at least one ion with a partially filled sub-shell of d electrons. – In period 4 only Ti-Cu are TM’s! – Note that when d block elements form ions the s electrons are lost first ...
... • For this reason, a transition metal is defined as being an element which forms at least one ion with a partially filled sub-shell of d electrons. – In period 4 only Ti-Cu are TM’s! – Note that when d block elements form ions the s electrons are lost first ...
as a PDF
... Both ligand field effects and inter-electronic repulsion produce irregularities in the chemistry of transition series. Irregularities due to inter-electronic repulsion are most obvious in the lanthanide series where ligand field effects are very small. For the first century of lanthanide chemistry, ...
... Both ligand field effects and inter-electronic repulsion produce irregularities in the chemistry of transition series. Irregularities due to inter-electronic repulsion are most obvious in the lanthanide series where ligand field effects are very small. For the first century of lanthanide chemistry, ...
{Ru(trpy)(bpy)} (trpy ) 2,2
... to utilize DMcT2- as a mediator of the electronic communication. The ligand has Lewis base centers on the thiadiazole ring, which can be utilized as the proton acceptor even in the bridging form. We have now prepared a new dimeric complex, [{Ru(trpy)(bpy)}2(µ-S,S′-DMcT)]2+ (bpy ) 2,2′-bipyridine and ...
... to utilize DMcT2- as a mediator of the electronic communication. The ligand has Lewis base centers on the thiadiazole ring, which can be utilized as the proton acceptor even in the bridging form. We have now prepared a new dimeric complex, [{Ru(trpy)(bpy)}2(µ-S,S′-DMcT)]2+ (bpy ) 2,2′-bipyridine and ...
Encapsulated pyridazine Cr(III) complexes prepared from
... diffusion of functionalized ligands into the zeolite through the pores was promoted, where form complexes with the intrazeolite metal ion, obtained by biosorption method [10-11]. Cr(III) complex with pyridazine ligand is typically four coordinate with a planar geometry around the metal centre. This ...
... diffusion of functionalized ligands into the zeolite through the pores was promoted, where form complexes with the intrazeolite metal ion, obtained by biosorption method [10-11]. Cr(III) complex with pyridazine ligand is typically four coordinate with a planar geometry around the metal centre. This ...
complexes of transition metals with uramildiacetic acid
... have improved the method of preparation and extended the determinations of stability constants to cover the series of alkali metals (Li+, Na+, K+), thallium(I) and alkaline-earth metals (Be 2 +, Mg 2 +, Ca'+, Sr 2 +, Ba 2 +). It has been shown that the complexes of the alkali metals are stabilized b ...
... have improved the method of preparation and extended the determinations of stability constants to cover the series of alkali metals (Li+, Na+, K+), thallium(I) and alkaline-earth metals (Be 2 +, Mg 2 +, Ca'+, Sr 2 +, Ba 2 +). It has been shown that the complexes of the alkali metals are stabilized b ...
Coordination compounds in nature
... retain transition metal cations which have been arranged below according to the decreasing ability for chelating Fe3+>Cu2+>Ni2+>Co2+>Zn2+>Fe2+>Mn2+ The chemical reactions in cation exchange and chelation make it possible for calcium and the other elements to be changed into water-soluble forms that ...
... retain transition metal cations which have been arranged below according to the decreasing ability for chelating Fe3+>Cu2+>Ni2+>Co2+>Zn2+>Fe2+>Mn2+ The chemical reactions in cation exchange and chelation make it possible for calcium and the other elements to be changed into water-soluble forms that ...
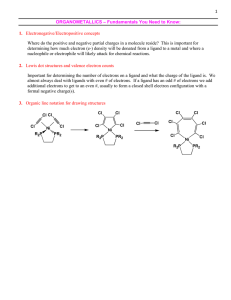
Organometallic Chemistry
... * The strength of this bonding depends on several factors, including the charge on the complex and the ligand environment of the metal. * If this picture of bonding between CO and metal atoms is correct, it should be supported by experimental evidence. Two sources of such evidence are infrared spect ...
... * The strength of this bonding depends on several factors, including the charge on the complex and the ligand environment of the metal. * If this picture of bonding between CO and metal atoms is correct, it should be supported by experimental evidence. Two sources of such evidence are infrared spect ...
Complexes of Ethylenediaminetetracarboxylate Ligands
... mechanics (LFMM)39 developed by one of the current authors. Both offer the possibility of rapid, accurate calculations of the structural and spectral properties of coordination complexes. However, both approaches are parametric, and we are not aware of any existing force-field parameters for copper(II ...
... mechanics (LFMM)39 developed by one of the current authors. Both offer the possibility of rapid, accurate calculations of the structural and spectral properties of coordination complexes. However, both approaches are parametric, and we are not aware of any existing force-field parameters for copper(II ...
Rhenium-Carbon Bonding in ErzReCz, an Organometallic Polymer
... along the chain. The middle carbon to Re distances are 2.03 and 1.97 A, respectively, while the terminal carbon to Re distance is 1.93 A. These separations are comparable to an estimated distance for a Re=C double bond.2 The Re atom in the ternary compound is in a distorted trigonal planar coordinat ...
... along the chain. The middle carbon to Re distances are 2.03 and 1.97 A, respectively, while the terminal carbon to Re distance is 1.93 A. These separations are comparable to an estimated distance for a Re=C double bond.2 The Re atom in the ternary compound is in a distorted trigonal planar coordinat ...
Chemical Bonding Ionic bonds result from an electrostatic attraction
... When phosphorus reacts with excess chlorine gas, the compound phosphorus pentachloride (PCl5) is formed. In the gaseous and liquid state, this substance consists of PCl5 molecules, but in the solid state it consists of a 1:1 misture of PCl4+ an PCl6- ions. Predict the geometric structures of PCl5, P ...
... When phosphorus reacts with excess chlorine gas, the compound phosphorus pentachloride (PCl5) is formed. In the gaseous and liquid state, this substance consists of PCl5 molecules, but in the solid state it consists of a 1:1 misture of PCl4+ an PCl6- ions. Predict the geometric structures of PCl5, P ...
Spin crossover

Spin Crossover (SCO), sometimes referred to as spin transition or spin equilibrium behavior, is a phenomenon that occurs in some metal complexes wherein the spin state of the complex changes due to external stimuli such as a variation of temperature, pressure, light irradiation or an influence of a magnetic field.With regard to a ligand field and ligand field theory, the change in spin state is a transition from a low spin (LS) ground state electron configuration to a high spin (HS) ground state electron configuration of the metal’s d atomic orbitals (AOs), or vice versa. The magnitude of the ligand field splitting along with the pairing energy of the complex determines whether it will have a LS or HS electron configuration. A LS state occurs because the ligand field splitting (Δ) is greater than the pairing energy of the complex (which is an unfavorable process).Figure 1 is a simplified illustration of the metal’s d orbital splitting in the presence of an octahedral ligand field. A large splitting between the t2g and eg AOs requires a substantial amount of energy for the electrons to overcome the energy gap (Δ) to comply with Hund’s Rule. Therefore, electrons will fill the lower energy t2g orbitals completely before populating the higher energy eg orbitals. Conversely, a HS state occurs with weaker ligand fields and smaller orbital splitting. In this case the energy required to populate the higher levels is substantially less than the pairing energy and the electrons fill the orbitals according to Hund’s Rule by populating the higher energy orbitals before pairing with electrons in the lower lying orbitals. An example of a metal ion that can exist in either a LS or HS state is Fe3+ in an octahedral ligand field. Depending on the ligands that are coordinated to this complex the Fe3+ can attain a LS or a HS state, as in Figure 1.Spin crossover refers to the transitions between high to low, or low to high, spin states. This phenomenon is commonly observed with some first row transition metal complexes with a d4 through d7 electron configuration in an octahedral ligand geometry. Spin transition curves are a common representation of SCO phenomenon with the most commonly observed types depicted in Figure 2 in which γHS (the high-spin molar fraction) is plotted vs. T. The figure shows a gradual spin transition (left), an abrupt transition with hysteresis (middle) and a two-step transition (right). For a transition to be considered gradual, it typically takes place over a large temperature range, even up to several hundred K, whereas for a transition to be considered abrupt, it should take place within 10 K or less.These curves indicate that a spin transition has occurred in a metal complex as temperature changed. The gradual transition curve is an indication that not all metal centers within the complex are undergoing the transition at the same temperature. The abrupt spin change with hysteresis indicates a strong cooperativity, or “communication”, between neighboring metal complexes. In the latter case, the material is bistable and can exist in the two different spin states with a different range of external stimuli (temperature in this case) for the two phenomena, namely LS → HS and HS → LS. The two-step transition is relatively rare but is observed, for example, with dinuclear SCO complexes for which the spin transition in one metal center renders the transition in the second metal center less favorable.There are several types of spin crossover that can occur in a complex; some of them are light induced excited state spin trapping (LIESST), ligand-driven light induced spin change (LD-LISC), and charge transfer induced spin transition (CTIST).