Document
... * Complex ions of the same central atom but different ligands have different colours. Colours of various complex ions formed by copper(II) ion are listed below : ...
... * Complex ions of the same central atom but different ligands have different colours. Colours of various complex ions formed by copper(II) ion are listed below : ...
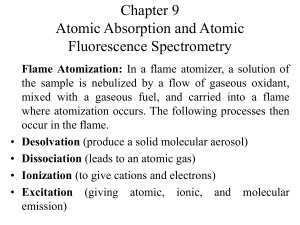
Chapter 9 Atomic Absorption and Atomic Fluorescence Spectrometry
... It provides enhanced sensitivity because the entire sample is atomized in a short period, and the average residence time of the atoms in the optical path is a second or more. A few microliters of sample are first evaporated at a low temperature and then ashed at a somewhat higher temperature in an e ...
... It provides enhanced sensitivity because the entire sample is atomized in a short period, and the average residence time of the atoms in the optical path is a second or more. A few microliters of sample are first evaporated at a low temperature and then ashed at a somewhat higher temperature in an e ...
Chapter 1 Introduction
... compared, it will usually be found that the sizes of the characteristic plane ―faces‖ are not in the same proportion the ―habit‖ varies from crystal to crystal‖. On the other hand, the interfacial angles are always the same for crystals of a given material. ...
... compared, it will usually be found that the sizes of the characteristic plane ―faces‖ are not in the same proportion the ―habit‖ varies from crystal to crystal‖. On the other hand, the interfacial angles are always the same for crystals of a given material. ...
Chapter 27, Nickel, Palladium and Platinum
... produce an axial polarization of the metal and ligand lone pair inducing a positive charge on the near-side of the metal and a negative charge on the far-side which weakens the trans-ligand attachment to the metal. ...
... produce an axial polarization of the metal and ligand lone pair inducing a positive charge on the near-side of the metal and a negative charge on the far-side which weakens the trans-ligand attachment to the metal. ...
Comparison for Bonding Situation between Tetrylone and Tetrylene
... Ψk/Ψ-k indicated the sign of the orbitals, and the yellow/blue colors in the deformation density designated charge depletion, and the blue areas pointed to charge accumulation. The charge flow occurs in the direction from yellow to blue. Figure 2a and 2d gave the NOCV pairs Ψ1/Ψ-1 and the defo ...
... Ψk/Ψ-k indicated the sign of the orbitals, and the yellow/blue colors in the deformation density designated charge depletion, and the blue areas pointed to charge accumulation. The charge flow occurs in the direction from yellow to blue. Figure 2a and 2d gave the NOCV pairs Ψ1/Ψ-1 and the defo ...
International Journal of Mass Spectrometry
... in the fingerprint region. The carboxyl carbonyl stretching band near 1715 cm−1 and the Amide II peak near 1500 cm−1 are well matched, while the Amide I peak seen at about 1670 cm−1 is predicted about 20 cm−1 too low, which is not an unacceptable degree of error. The predicted spectra for the alterna ...
... in the fingerprint region. The carboxyl carbonyl stretching band near 1715 cm−1 and the Amide II peak near 1500 cm−1 are well matched, while the Amide I peak seen at about 1670 cm−1 is predicted about 20 cm−1 too low, which is not an unacceptable degree of error. The predicted spectra for the alterna ...
Pdf - Text of NPTEL IIT Video Lectures
... electron density from this particular oxygen is pushed towards the vacant orbital of iron and the p k a basically, the p k a of this O H is changing. And this particular p k a value is such that, it is going to towards some position, where we can consider that this bound water molecules. These bound ...
... electron density from this particular oxygen is pushed towards the vacant orbital of iron and the p k a basically, the p k a of this O H is changing. And this particular p k a value is such that, it is going to towards some position, where we can consider that this bound water molecules. These bound ...
Synthesis and Characterization of Mononuclear, Pseudotetrahedral
... the analogous zinc(II) and cobalt(III) compounds.18 The shift to more positive potentials in the gallium(III) tropocoronands relative to the zinc(II) analogues can be attributed to the higher oxidation state of the gallium center. Ligand oxidation in the gallium complexes may occur at more positive ...
... the analogous zinc(II) and cobalt(III) compounds.18 The shift to more positive potentials in the gallium(III) tropocoronands relative to the zinc(II) analogues can be attributed to the higher oxidation state of the gallium center. Ligand oxidation in the gallium complexes may occur at more positive ...
Photochemistry of tetrasulfido complexes of molybdenum (VI
... ligands to the metal.' However, a t these high formal oxidation states of the metal, the bonding in these complexes must have large covalent contributions. I t follows that some of the MO's are strongly delocalized between metal and ligands. L M C T transitions may then not be associated with the tr ...
... ligands to the metal.' However, a t these high formal oxidation states of the metal, the bonding in these complexes must have large covalent contributions. I t follows that some of the MO's are strongly delocalized between metal and ligands. L M C T transitions may then not be associated with the tr ...
Reversible lithium uptake by CoP3 at low potential: role of the anion
... phosphide, CoP3 , provides a reversible capacity of 400 mAh/g at an average potential of 0.9 V vs Li=Liþ , via a novel mechanism. The latter was revealed using a combination of X-ray diffraction, electron microscopy, and potentiodynamic and galvanostatic intermittent measurements coupled with X-ray p ...
... phosphide, CoP3 , provides a reversible capacity of 400 mAh/g at an average potential of 0.9 V vs Li=Liþ , via a novel mechanism. The latter was revealed using a combination of X-ray diffraction, electron microscopy, and potentiodynamic and galvanostatic intermittent measurements coupled with X-ray p ...
i
... not known. The synthesis of red-black 1 consists of generating Li3[HIPTN3N] in situ from H3[HIPTN3N] and 3 equiv of LiN(TMS)2 in ether, adding CrCl3, and stirring the heterogeneous mixture for 2 days. Compound 1 is converted into 2 upon addition of THF to a solution of 1, while application of heat a ...
... not known. The synthesis of red-black 1 consists of generating Li3[HIPTN3N] in situ from H3[HIPTN3N] and 3 equiv of LiN(TMS)2 in ether, adding CrCl3, and stirring the heterogeneous mixture for 2 days. Compound 1 is converted into 2 upon addition of THF to a solution of 1, while application of heat a ...
Improving our understanding of molecular geometry and the VSEPR
... importance of ligand – ligand repulsions and the study of electron density distributions. These improvements have enabled several aspects of the VSEPR model to be better understood and explanations of exceptions to the model to be given. In this paper I review these new developments and their relati ...
... importance of ligand – ligand repulsions and the study of electron density distributions. These improvements have enabled several aspects of the VSEPR model to be better understood and explanations of exceptions to the model to be given. In this paper I review these new developments and their relati ...
Bent Cis do MOO^^` vs. Linear Trans dofo UOz2+: A Significant Role
... by 6p, compared with a l and bl of cis, which in turn results in a more effective stabilization of uu+ by 5f. The considerations we present here are based on calculations without inclusion of spin-orbit interactions. The effects of (9) The z axis is along 0-U-0 ...
... by 6p, compared with a l and bl of cis, which in turn results in a more effective stabilization of uu+ by 5f. The considerations we present here are based on calculations without inclusion of spin-orbit interactions. The effects of (9) The z axis is along 0-U-0 ...
Spin crossover

Spin Crossover (SCO), sometimes referred to as spin transition or spin equilibrium behavior, is a phenomenon that occurs in some metal complexes wherein the spin state of the complex changes due to external stimuli such as a variation of temperature, pressure, light irradiation or an influence of a magnetic field.With regard to a ligand field and ligand field theory, the change in spin state is a transition from a low spin (LS) ground state electron configuration to a high spin (HS) ground state electron configuration of the metal’s d atomic orbitals (AOs), or vice versa. The magnitude of the ligand field splitting along with the pairing energy of the complex determines whether it will have a LS or HS electron configuration. A LS state occurs because the ligand field splitting (Δ) is greater than the pairing energy of the complex (which is an unfavorable process).Figure 1 is a simplified illustration of the metal’s d orbital splitting in the presence of an octahedral ligand field. A large splitting between the t2g and eg AOs requires a substantial amount of energy for the electrons to overcome the energy gap (Δ) to comply with Hund’s Rule. Therefore, electrons will fill the lower energy t2g orbitals completely before populating the higher energy eg orbitals. Conversely, a HS state occurs with weaker ligand fields and smaller orbital splitting. In this case the energy required to populate the higher levels is substantially less than the pairing energy and the electrons fill the orbitals according to Hund’s Rule by populating the higher energy orbitals before pairing with electrons in the lower lying orbitals. An example of a metal ion that can exist in either a LS or HS state is Fe3+ in an octahedral ligand field. Depending on the ligands that are coordinated to this complex the Fe3+ can attain a LS or a HS state, as in Figure 1.Spin crossover refers to the transitions between high to low, or low to high, spin states. This phenomenon is commonly observed with some first row transition metal complexes with a d4 through d7 electron configuration in an octahedral ligand geometry. Spin transition curves are a common representation of SCO phenomenon with the most commonly observed types depicted in Figure 2 in which γHS (the high-spin molar fraction) is plotted vs. T. The figure shows a gradual spin transition (left), an abrupt transition with hysteresis (middle) and a two-step transition (right). For a transition to be considered gradual, it typically takes place over a large temperature range, even up to several hundred K, whereas for a transition to be considered abrupt, it should take place within 10 K or less.These curves indicate that a spin transition has occurred in a metal complex as temperature changed. The gradual transition curve is an indication that not all metal centers within the complex are undergoing the transition at the same temperature. The abrupt spin change with hysteresis indicates a strong cooperativity, or “communication”, between neighboring metal complexes. In the latter case, the material is bistable and can exist in the two different spin states with a different range of external stimuli (temperature in this case) for the two phenomena, namely LS → HS and HS → LS. The two-step transition is relatively rare but is observed, for example, with dinuclear SCO complexes for which the spin transition in one metal center renders the transition in the second metal center less favorable.There are several types of spin crossover that can occur in a complex; some of them are light induced excited state spin trapping (LIESST), ligand-driven light induced spin change (LD-LISC), and charge transfer induced spin transition (CTIST).