chapter15
... • Most transition metal complexes are brightly colored • Exception: those with empty d sublevels (e.g., Sc3+; those with full d sublevels (e.g., Zn2+) • The splitting of the d sublevel results in an energy difference that corresponds to the visible region of the electromagnetic spectrum • Visible li ...
... • Most transition metal complexes are brightly colored • Exception: those with empty d sublevels (e.g., Sc3+; those with full d sublevels (e.g., Zn2+) • The splitting of the d sublevel results in an energy difference that corresponds to the visible region of the electromagnetic spectrum • Visible li ...
+1 0
... momentum for dn configuration ML = ∑ml • Spin momentum of individual electron spins couple together to give total spin, S = ∑s • Inter-electronic repulsions between the electrons in the d orbitals give rise to ground state and excited states for dn configurations. • States are labeled with Tern Symb ...
... momentum for dn configuration ML = ∑ml • Spin momentum of individual electron spins couple together to give total spin, S = ∑s • Inter-electronic repulsions between the electrons in the d orbitals give rise to ground state and excited states for dn configurations. • States are labeled with Tern Symb ...
File
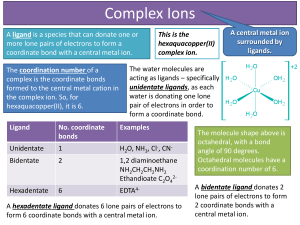
... A ligand is a species that can donate one or more lone pairs of electrons to form a coordinate bond with a central metal ion. The coordination number of a complex is the coordinate bonds formed to the central metal cation in the complex ion. So, for hexaquacopper(II), it is 6. ...
... A ligand is a species that can donate one or more lone pairs of electrons to form a coordinate bond with a central metal ion. The coordination number of a complex is the coordinate bonds formed to the central metal cation in the complex ion. So, for hexaquacopper(II), it is 6. ...
Lab 11
... number of bonds it can form with ligands – typically 1, 2, 3, 4, 5, or 6. The coordination number of Fe3+ is 6. ...
... number of bonds it can form with ligands – typically 1, 2, 3, 4, 5, or 6. The coordination number of Fe3+ is 6. ...
Lecture 2
... Some schematic diagrams showing how p bonding occurs with a ligand having a d orbital (such as in P), or a p* orbital, or a vacant p orbital. ...
... Some schematic diagrams showing how p bonding occurs with a ligand having a d orbital (such as in P), or a p* orbital, or a vacant p orbital. ...
Introduction to Magnetochemistry
... The processes which create magnetic fields in an atom are 1. Nuclear spin. Some nuclei, such as a hydrogen atom, have a net spin, which creates a magnetic field. 2. Electron spin. An electron has two intrinsic spin states (similar to a top spinning) which we call up and down or alpha and beta. 3. El ...
... The processes which create magnetic fields in an atom are 1. Nuclear spin. Some nuclei, such as a hydrogen atom, have a net spin, which creates a magnetic field. 2. Electron spin. An electron has two intrinsic spin states (similar to a top spinning) which we call up and down or alpha and beta. 3. El ...
Chemguide – answers TRANSITION METALS: GENERAL FEATURES
... If you choose any other examples, you will have to check them against your source. b) (i) The ionisation energy is only one of a number of energy steps involved in forming compounds containing the ions, although it is the most important energy input term. Energy is then released when the positive io ...
... If you choose any other examples, you will have to check them against your source. b) (i) The ionisation energy is only one of a number of energy steps involved in forming compounds containing the ions, although it is the most important energy input term. Energy is then released when the positive io ...
Chapter 1: Fundamental Concepts
... • The oxidation state of the metal (charge!) D is greater for M3+ than for M2+ • The row of the metal in the periodic table (size!) for a given ligand and oxidation state of the metal, D increases going down in a group e.g. D is greater in Ru(NH3)63+ than in Fe(NH3)63+ Colors of metal complexes are ...
... • The oxidation state of the metal (charge!) D is greater for M3+ than for M2+ • The row of the metal in the periodic table (size!) for a given ligand and oxidation state of the metal, D increases going down in a group e.g. D is greater in Ru(NH3)63+ than in Fe(NH3)63+ Colors of metal complexes are ...
Molecular orbital approach to bonding in octahedral complexes, ML 6
... Consequences of P-bonding interactions between metal and ligand Enhanced D-splitting for P-acceptor ligands makes P-unsaturated ligands like CO, CN- and alkenes very strong-field ligands. Stabilization of metals in low oxidation states. Delocalization of electron density from low oxidation sta ...
... Consequences of P-bonding interactions between metal and ligand Enhanced D-splitting for P-acceptor ligands makes P-unsaturated ligands like CO, CN- and alkenes very strong-field ligands. Stabilization of metals in low oxidation states. Delocalization of electron density from low oxidation sta ...
Chapter 1 Structure and Bonding
... a) Each bond between metals counts 1 electron per metal: Mn—Mn = 1 eb) Total ligand charge = 0, so Mn0 = d7 c) 5 CO ligands per metal = 10 electrons for a total of 18 electrons per Mn ...
... a) Each bond between metals counts 1 electron per metal: Mn—Mn = 1 eb) Total ligand charge = 0, so Mn0 = d7 c) 5 CO ligands per metal = 10 electrons for a total of 18 electrons per Mn ...
Spin crossover

Spin Crossover (SCO), sometimes referred to as spin transition or spin equilibrium behavior, is a phenomenon that occurs in some metal complexes wherein the spin state of the complex changes due to external stimuli such as a variation of temperature, pressure, light irradiation or an influence of a magnetic field.With regard to a ligand field and ligand field theory, the change in spin state is a transition from a low spin (LS) ground state electron configuration to a high spin (HS) ground state electron configuration of the metal’s d atomic orbitals (AOs), or vice versa. The magnitude of the ligand field splitting along with the pairing energy of the complex determines whether it will have a LS or HS electron configuration. A LS state occurs because the ligand field splitting (Δ) is greater than the pairing energy of the complex (which is an unfavorable process).Figure 1 is a simplified illustration of the metal’s d orbital splitting in the presence of an octahedral ligand field. A large splitting between the t2g and eg AOs requires a substantial amount of energy for the electrons to overcome the energy gap (Δ) to comply with Hund’s Rule. Therefore, electrons will fill the lower energy t2g orbitals completely before populating the higher energy eg orbitals. Conversely, a HS state occurs with weaker ligand fields and smaller orbital splitting. In this case the energy required to populate the higher levels is substantially less than the pairing energy and the electrons fill the orbitals according to Hund’s Rule by populating the higher energy orbitals before pairing with electrons in the lower lying orbitals. An example of a metal ion that can exist in either a LS or HS state is Fe3+ in an octahedral ligand field. Depending on the ligands that are coordinated to this complex the Fe3+ can attain a LS or a HS state, as in Figure 1.Spin crossover refers to the transitions between high to low, or low to high, spin states. This phenomenon is commonly observed with some first row transition metal complexes with a d4 through d7 electron configuration in an octahedral ligand geometry. Spin transition curves are a common representation of SCO phenomenon with the most commonly observed types depicted in Figure 2 in which γHS (the high-spin molar fraction) is plotted vs. T. The figure shows a gradual spin transition (left), an abrupt transition with hysteresis (middle) and a two-step transition (right). For a transition to be considered gradual, it typically takes place over a large temperature range, even up to several hundred K, whereas for a transition to be considered abrupt, it should take place within 10 K or less.These curves indicate that a spin transition has occurred in a metal complex as temperature changed. The gradual transition curve is an indication that not all metal centers within the complex are undergoing the transition at the same temperature. The abrupt spin change with hysteresis indicates a strong cooperativity, or “communication”, between neighboring metal complexes. In the latter case, the material is bistable and can exist in the two different spin states with a different range of external stimuli (temperature in this case) for the two phenomena, namely LS → HS and HS → LS. The two-step transition is relatively rare but is observed, for example, with dinuclear SCO complexes for which the spin transition in one metal center renders the transition in the second metal center less favorable.There are several types of spin crossover that can occur in a complex; some of them are light induced excited state spin trapping (LIESST), ligand-driven light induced spin change (LD-LISC), and charge transfer induced spin transition (CTIST).