Mechanisms of Oxidation with Oxygen
... Zn(CH3) 2 (21) at sufficiently low pressure of the reactants, a rather clean reaction to form solid intermediate products can be isolated. However, the kinetic behavior again suggests that the reaction takes place on the surface, and m a y in addition be a chain reaction. The possibility of a simple ...
... Zn(CH3) 2 (21) at sufficiently low pressure of the reactants, a rather clean reaction to form solid intermediate products can be isolated. However, the kinetic behavior again suggests that the reaction takes place on the surface, and m a y in addition be a chain reaction. The possibility of a simple ...
unit 17 organic compounds containing oxygen and nitrogen atoms
... As you know, ni~cleopliilicadditionreaction involves addition of a nucleopliile to the partially positively charged carbon atom of tlie carbonyl group. Tlie relative reactivities of carbonyl group in ni~cleophilicaddition reactions may be attributed partly to the extent of polarisatiou of tlie carbo ...
... As you know, ni~cleopliilicadditionreaction involves addition of a nucleopliile to the partially positively charged carbon atom of tlie carbonyl group. Tlie relative reactivities of carbonyl group in ni~cleophilicaddition reactions may be attributed partly to the extent of polarisatiou of tlie carbo ...
final1-final_report
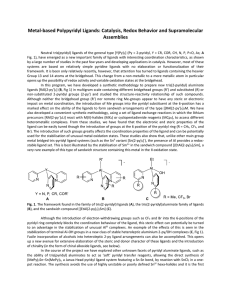
... Neutral tris(pyridyl) ligands of the general type [Y(Py) 3] (Py = 2-pyridyl, Y = CR, COR, CH, N, P, P=O, As; A Fig. 1), have emerged as a new important family of ligands with interesting coordination characteristics, as shown by a large number of studies in the past few years and developing applicat ...
... Neutral tris(pyridyl) ligands of the general type [Y(Py) 3] (Py = 2-pyridyl, Y = CR, COR, CH, N, P, P=O, As; A Fig. 1), have emerged as a new important family of ligands with interesting coordination characteristics, as shown by a large number of studies in the past few years and developing applicat ...
CHAPTER 1 INTRODUCTION 1.1 Research Background
... association between these two ligand systems. These calculations have shown that the sterically demanding carbenes are infact bulkier than P(tBu)3 [11]. Effect of ancillary ligands is also required with the steric hindrance of the labile substituents attached to the central metal. ...
... association between these two ligand systems. These calculations have shown that the sterically demanding carbenes are infact bulkier than P(tBu)3 [11]. Effect of ancillary ligands is also required with the steric hindrance of the labile substituents attached to the central metal. ...
Ring-closing metathesis

Ring-closing metathesis, or RCM, is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.The most commonly synthesized ring sizes are between 5-7 atoms; however, reported syntheses include 45- up to 90- membered macroheterocycles. These reactions are metal-catalyzed and proceed through a metallacyclobutane intermediate. It was first published by Dider Villemin in 1980 describing the synthesis of an Exaltolide precursor, and later become popularized by Robert H. Grubbs and Richard R. Schrock, who shared the Nobel Prize in Chemistry, along with Yves Chauvin, in 2005 for their combined work in olefin metathesis. RCM is a favorite among organic chemists due to its synthetic utility in the formation of rings, which were previously difficult to access efficiently, and broad substrate scope. Since the only major by-product is ethylene, these reactions may also be considered atom economic, an increasingly important concern in the development of green chemistry.There are several reviews published on ring-closing metathesis.