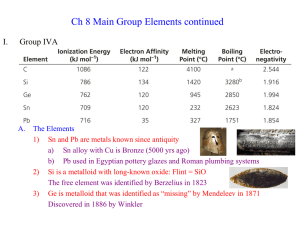
C1a Revision notes - Calthorpe Park Moodle
... a mixture into a number of different parts, called fractions. A tall column is fitted above the mixture, with several condensers coming off at different heights. The column is hot at the bottom and cool at the top. Substances with high boiling points condense at the bottom and substances with low bo ...
... a mixture into a number of different parts, called fractions. A tall column is fitted above the mixture, with several condensers coming off at different heights. The column is hot at the bottom and cool at the top. Substances with high boiling points condense at the bottom and substances with low bo ...
15.2 Electrons and Chemical Bonds
... Example, carbon To see how this works, consider the compound carbon tetrachloride tetrachloride (CCl4). Carbon has an oxidation number of 4+. Chlorine has an oxidation number of 1-. It takes four chlorine atoms to cancel with carbon’s 4+ oxidation number. ...
... Example, carbon To see how this works, consider the compound carbon tetrachloride tetrachloride (CCl4). Carbon has an oxidation number of 4+. Chlorine has an oxidation number of 1-. It takes four chlorine atoms to cancel with carbon’s 4+ oxidation number. ...
PDF of this page
... The mathematic and conceptual foundations of physical chemistry will be introduced with an over-arching theme of determination of energy allocation within atomic and molecular systems. Topics will include determination and measurement of energy states in atoms and molecules, simple quantum mechanica ...
... The mathematic and conceptual foundations of physical chemistry will be introduced with an over-arching theme of determination of energy allocation within atomic and molecular systems. Topics will include determination and measurement of energy states in atoms and molecules, simple quantum mechanica ...
Print out Reviews # 1 through # 17
... 1. How is an element’s outer electron configuration related to its position on the Periodic Table? 2. What are the symbols for all of the elements that have the following outer electron configuration? (A) s1 (B) s2 p2 (C) s2 p5 3. Indicate which element in each of the following pairs has the larger ...
... 1. How is an element’s outer electron configuration related to its position on the Periodic Table? 2. What are the symbols for all of the elements that have the following outer electron configuration? (A) s1 (B) s2 p2 (C) s2 p5 3. Indicate which element in each of the following pairs has the larger ...
Theoretical Competition - Austrian Chemistry Olympiad
... Boron forms with phosphorus the binary compounds BP and B12P2. BP is generated at 900-1000°C from boron and white phosphorus P4. It is an extremely hard, chemically inert, and heat resistant substance. It exhibits a zinc blende structure: the boron atoms form a cubic face centred lattice. The P-atom ...
... Boron forms with phosphorus the binary compounds BP and B12P2. BP is generated at 900-1000°C from boron and white phosphorus P4. It is an extremely hard, chemically inert, and heat resistant substance. It exhibits a zinc blende structure: the boron atoms form a cubic face centred lattice. The P-atom ...
PDF of this page
... CHEM 1010. Introductory Chemistry for Environmental Studies. 4 Hours. A chemistry course with a focus on real-world societal issues. Students will develop critical thinking skills and an appreciation for the theoretical and practical aspects of chemistry while learning the fundamentals of chemistry. ...
... CHEM 1010. Introductory Chemistry for Environmental Studies. 4 Hours. A chemistry course with a focus on real-world societal issues. Students will develop critical thinking skills and an appreciation for the theoretical and practical aspects of chemistry while learning the fundamentals of chemistry. ...
InorgCh8.2
... a) Low bond energy in F2 makes it more reactive than expected i. Extrapolate from others to 290 kJ/mol; actually 159 kJ/mol ii. Repulsion of lone pairs responsible; also has long 143 vs. 128 pm bond iii. MO explanation: poor overlap of small orbitals b) HF weak acid: stronger H—F bonds not easily di ...
... a) Low bond energy in F2 makes it more reactive than expected i. Extrapolate from others to 290 kJ/mol; actually 159 kJ/mol ii. Repulsion of lone pairs responsible; also has long 143 vs. 128 pm bond iii. MO explanation: poor overlap of small orbitals b) HF weak acid: stronger H—F bonds not easily di ...
Chapter 3
... View of Elements and Compounds • Atomic elements exist in nature with single atoms as their basic units. Most elements fall into this category. • Examples are Na, Ne, C, K, Mg, etc. ...
... View of Elements and Compounds • Atomic elements exist in nature with single atoms as their basic units. Most elements fall into this category. • Examples are Na, Ne, C, K, Mg, etc. ...
Ionic Compounds
... View of Elements and Compounds • Atomic elements exist in nature with single atoms as their basic units. Most elements fall into this category. • Examples are Na, Ne, C, K, Mg, etc. ...
... View of Elements and Compounds • Atomic elements exist in nature with single atoms as their basic units. Most elements fall into this category. • Examples are Na, Ne, C, K, Mg, etc. ...
compounds - Belle Vernon Area
... View of Elements and Compounds • Atomic elements exist in nature with single atoms as their basic units. Most elements fall into this category. • Examples are Na, Ne, C, K, Mg, etc. ...
... View of Elements and Compounds • Atomic elements exist in nature with single atoms as their basic units. Most elements fall into this category. • Examples are Na, Ne, C, K, Mg, etc. ...
Directed Reading
... a. Helium does not react with other substances but does form new substances. b. Helium reacts with other substances but does not form new substances. c. Helium reacts with other substances to form new substances. d. Helium does not react with other substances to form new substances. ______ 9. A subs ...
... a. Helium does not react with other substances but does form new substances. b. Helium reacts with other substances but does not form new substances. c. Helium reacts with other substances to form new substances. d. Helium does not react with other substances to form new substances. ______ 9. A subs ...
Reversible and irreversible reactions - Chemwiki
... For e.g., potassium chlorate decomposes on heating to form potassium chloride and oxygen. ...
... For e.g., potassium chlorate decomposes on heating to form potassium chloride and oxygen. ...
Science 10 - SharpSchool
... 1. metals are good conductors, strong, malleable (pound into thin sheet), ductile (can draw into a wire, bendable), have high luster; are found on left side of stair case 2. non metals are poor conductors, non-lustrous, weak, etc…opposite properties to metals; found on right side of ...
... 1. metals are good conductors, strong, malleable (pound into thin sheet), ductile (can draw into a wire, bendable), have high luster; are found on left side of stair case 2. non metals are poor conductors, non-lustrous, weak, etc…opposite properties to metals; found on right side of ...
Honors Chemistry Curr
... Explain the roles of Mendeleev and Moseley in the development of the periodic table Describe the modern periodic table Explain how the periodic law can be used to predict the physical and chemical properties of elements Describe the periodic trends of atomic radius, ionization energy, electron affin ...
... Explain the roles of Mendeleev and Moseley in the development of the periodic table Describe the modern periodic table Explain how the periodic law can be used to predict the physical and chemical properties of elements Describe the periodic trends of atomic radius, ionization energy, electron affin ...
Honors Chemistry
... Explain the roles of Mendeleev and Moseley in the development of the periodic table Describe the modern periodic table Explain how the periodic law can be used to predict the physical and chemical properties of elements Describe the periodic trends of atomic radius, ionization energy, electron affin ...
... Explain the roles of Mendeleev and Moseley in the development of the periodic table Describe the modern periodic table Explain how the periodic law can be used to predict the physical and chemical properties of elements Describe the periodic trends of atomic radius, ionization energy, electron affin ...
CHEMISTRY
... Concerned with the rigorous treatment of equilibria that are of analytical importance and with an introduction into electro analytical methods, emission and absorption spectrophotometry, and modern separation methods, particularly chromatography. Prerequisite: CHEM 0120 or CHEM 0970. Corequisite: CH ...
... Concerned with the rigorous treatment of equilibria that are of analytical importance and with an introduction into electro analytical methods, emission and absorption spectrophotometry, and modern separation methods, particularly chromatography. Prerequisite: CHEM 0120 or CHEM 0970. Corequisite: CH ...
bioinorganic 1
... usually coupled to bond making-breaking). Few naturally occurring organic substrates can do this. Transition metals are excellent for electron transfer because they can adopt more than one oxidation state. Iron is particularly adept at this because the redox potential can be controlled by its enviro ...
... usually coupled to bond making-breaking). Few naturally occurring organic substrates can do this. Transition metals are excellent for electron transfer because they can adopt more than one oxidation state. Iron is particularly adept at this because the redox potential can be controlled by its enviro ...
unit 7 h chem notes - chemical equations
... II. Sometimes it is necessary to abbreviate the “phase” of the substance to the lower right of the substance. Some abbreviations are: s = solid, l= liquid, g ( )= gas, aq= aqueous, ppt ( )= precipitate. III Write equations using correct formulas of diatomic molecules, then Balance the equation for e ...
... II. Sometimes it is necessary to abbreviate the “phase” of the substance to the lower right of the substance. Some abbreviations are: s = solid, l= liquid, g ( )= gas, aq= aqueous, ppt ( )= precipitate. III Write equations using correct formulas of diatomic molecules, then Balance the equation for e ...
PowerPoint material for lecture 1 (September 4, 2012)
... • In bulk solids, all molecules are surrounded by and bound to neighboring atoms and the forces are in balance. Surface atoms are bound only on one side, leaving unbalanced atomic and molecular forces on the surface. These forces attract gases and molecules Van der Waals force, physical adsorpti ...
... • In bulk solids, all molecules are surrounded by and bound to neighboring atoms and the forces are in balance. Surface atoms are bound only on one side, leaving unbalanced atomic and molecular forces on the surface. These forces attract gases and molecules Van der Waals force, physical adsorpti ...
Balanced Chemical Equation
... • Elimination of the spectator ions results in a net ionic equation: ...
... • Elimination of the spectator ions results in a net ionic equation: ...
Inorganic chemistry

Inorganic chemistry deals with the synthesis and behavior of inorganic and organometallic compounds. This field covers all chemical compounds except the myriad organic compounds (carbon based compounds, usually containing C-H bonds), which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, and there is much overlap, most importantly in the sub-discipline of organometallic chemistry. It has applications in every aspect of the chemical industry–including catalysis, materials science, pigments, surfactants, coatings, medicine, fuel, and agriculture.