File
... means moles need to be converted to grams. Also we will need to find out the molar ration between Fe2O3 and Al. With all chemistry problems and most scientific problems you arrive at the answer by using logical steps rather than guessing. It’s important to set out your work neatly, step wise so that ...
... means moles need to be converted to grams. Also we will need to find out the molar ration between Fe2O3 and Al. With all chemistry problems and most scientific problems you arrive at the answer by using logical steps rather than guessing. It’s important to set out your work neatly, step wise so that ...
Today Electrochemistry electrons moving about equilibrium with a
... Mg2+ and O2- So the "oxidation state" of Mg is 2+ the "oxidation state" of O is 2- How will we figure it out for other molecules? There are rules. ...
... Mg2+ and O2- So the "oxidation state" of Mg is 2+ the "oxidation state" of O is 2- How will we figure it out for other molecules? There are rules. ...
Today Electrochemistry electrons moving about equilibrium with a
... How will we figure it out for other molecules?! There are rules.! ...
... How will we figure it out for other molecules?! There are rules.! ...
1 Lecture 11. Redox Chemistry Many elements in the periodic table
... Lecture 11. Redox Chemistry Many elements in the periodic table can exist in more than one oxidation state. Oxidation states are indicated by Roman numerals in parentheses (e.g. (+I), (-IV) etc.). The oxidation state represents the “electron content” of an element, which can be expressed as the exce ...
... Lecture 11. Redox Chemistry Many elements in the periodic table can exist in more than one oxidation state. Oxidation states are indicated by Roman numerals in parentheses (e.g. (+I), (-IV) etc.). The oxidation state represents the “electron content” of an element, which can be expressed as the exce ...
PPT Oxidation
... oxidation and reduction to reactions that do not formally involve the transfer of electrons. • CO(g) + H2O(g) CO2(g) + H2(g) • What changes in this reaction is the oxidation state of these atoms. The oxidation state of carbon increases from +2 to +4, while the oxidation state of the hydrogen decreas ...
... oxidation and reduction to reactions that do not formally involve the transfer of electrons. • CO(g) + H2O(g) CO2(g) + H2(g) • What changes in this reaction is the oxidation state of these atoms. The oxidation state of carbon increases from +2 to +4, while the oxidation state of the hydrogen decreas ...
Basic chemistry - Ross University
... to measure changes in enthalpy. For biochemical reactions we can thus use ∆H as an approximation for ∆E, which is the much more important parameter. Entropy (S) is a measure of chaos. If you look at fig. 1.1, you see two bodies of different temperature. If you bring them close together, heat could t ...
... to measure changes in enthalpy. For biochemical reactions we can thus use ∆H as an approximation for ∆E, which is the much more important parameter. Entropy (S) is a measure of chaos. If you look at fig. 1.1, you see two bodies of different temperature. If you bring them close together, heat could t ...
Chap. 4 - Chemical Reactions
... 2. Solid calcium reacts with oxygen gas. 3. Solutions of aluminum chloride & sodium carbonate are mixed. 4. Liquid magnesium bromide is decomposed at high temperature. 5. Solid nickel is reacted with aqueous magnesium sulfate. 6. Chlorine gas is reacted with aqueous potassium bromide. 7. Solid magne ...
... 2. Solid calcium reacts with oxygen gas. 3. Solutions of aluminum chloride & sodium carbonate are mixed. 4. Liquid magnesium bromide is decomposed at high temperature. 5. Solid nickel is reacted with aqueous magnesium sulfate. 6. Chlorine gas is reacted with aqueous potassium bromide. 7. Solid magne ...
PPT Oxidation
... oxidation and reduction to reactions that do not formally involve the transfer of electrons. • CO(g) + H2O(g) CO2(g) + H2(g) • What changes in this reaction is the oxidation state of these atoms. The oxidation state of carbon increases from +2 to +4, while the oxidation state of the hydrogen decreas ...
... oxidation and reduction to reactions that do not formally involve the transfer of electrons. • CO(g) + H2O(g) CO2(g) + H2(g) • What changes in this reaction is the oxidation state of these atoms. The oxidation state of carbon increases from +2 to +4, while the oxidation state of the hydrogen decreas ...
RxnTypesPrednotesIIAP
... All metallic sulfates form the metallic oxide and release sulfur trioxide gas, with the exception of the I-A and II-A metallic sulfates. The metallic sulfates of the alkali family and the alkaline earth family are so stable that heating has no effect. ex. FeSO4 ----- -----> FeO + SO3 K2SO4 ----- ...
... All metallic sulfates form the metallic oxide and release sulfur trioxide gas, with the exception of the I-A and II-A metallic sulfates. The metallic sulfates of the alkali family and the alkaline earth family are so stable that heating has no effect. ex. FeSO4 ----- -----> FeO + SO3 K2SO4 ----- ...
H 2
... compounds combine one Carbonate with water ion. as it isbalance shown above. to form acids. ...
... compounds combine one Carbonate with water ion. as it isbalance shown above. to form acids. ...
Introduction - Assets - Cambridge University Press
... Water molecules are widespread in the universe as grains of solid ice or as very dilute water vapor. Liquid water is a fleeting substance that can persist only above 0 °C and under an atmospheric pressure higher than 6 mbars. Therefore, the size of a planet and its distance from the star are two bas ...
... Water molecules are widespread in the universe as grains of solid ice or as very dilute water vapor. Liquid water is a fleeting substance that can persist only above 0 °C and under an atmospheric pressure higher than 6 mbars. Therefore, the size of a planet and its distance from the star are two bas ...
Your views are welcomed upon the theme of
... have this type of outer shell structure are seldom found in nature: so single atoms of carbon, oxygen, fluorine etc. are not detected in high levels. Under any kinds of ‘normal’ conditions these species would have a short life-life. Similarly ions which have this type of ‘noble gas electronic struct ...
... have this type of outer shell structure are seldom found in nature: so single atoms of carbon, oxygen, fluorine etc. are not detected in high levels. Under any kinds of ‘normal’ conditions these species would have a short life-life. Similarly ions which have this type of ‘noble gas electronic struct ...
Chapter 4 Student Notes
... A redox equation is balanced if: it is balanced for atoms on each side. the total electrons lost and gained are equal. A bit of terminology; the substance that is oxidized can be said to have caused the other substance to be reduced. It is the reducing agent. The substance that is reduced can be sai ...
... A redox equation is balanced if: it is balanced for atoms on each side. the total electrons lost and gained are equal. A bit of terminology; the substance that is oxidized can be said to have caused the other substance to be reduced. It is the reducing agent. The substance that is reduced can be sai ...
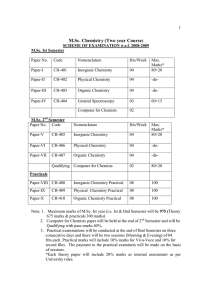
M.Sc. Chemistry (Two year Course)
... Schrodinger wave equation for a particle in a three dimensional box and the concept of degeneracy of energy levels. Schrodinger wave equation for linear harmonic oscillator, solution by polynomial method, zero point energy and its consequence. Schrodinger wave equation for three dimensional Rigid ro ...
... Schrodinger wave equation for a particle in a three dimensional box and the concept of degeneracy of energy levels. Schrodinger wave equation for linear harmonic oscillator, solution by polynomial method, zero point energy and its consequence. Schrodinger wave equation for three dimensional Rigid ro ...
Pharmaceutical Chemistry - International Medical University
... drug design and synthesis, drug formulation and testing, as well as the delivery of drugs in the body. In view of the increase in the occurrence of existing and new diseases, continuous work in the discovery of new drugs that have high therapeutic efficacies but minimal side effects is necessary. Ph ...
... drug design and synthesis, drug formulation and testing, as well as the delivery of drugs in the body. In view of the increase in the occurrence of existing and new diseases, continuous work in the discovery of new drugs that have high therapeutic efficacies but minimal side effects is necessary. Ph ...
Periodic Table of Elements
... • Elements become more stable as they gain more valence electrons. • As a result, atoms will gain, lose or share electrons to form compounds so that they have 8 valence electrons or a full shell. • This is called the Octet Rule. However there are many exceptions, but this is an easy way to predict c ...
... • Elements become more stable as they gain more valence electrons. • As a result, atoms will gain, lose or share electrons to form compounds so that they have 8 valence electrons or a full shell. • This is called the Octet Rule. However there are many exceptions, but this is an easy way to predict c ...
Net Ionic Prep Session NMSI INSTRUCTOR
... WRITE THESE DISSOCIATED except concentrated sulfuric, it really is 97% H2SO4 and 3% water in the jug, so water is way outnumbered and the molecules don’t dissociate completely. If carbonic acid is formed as a product Æ CO2 + H2O. 5. Strong bases: hydroxides [and oxides] of IA and IIA* metals—write t ...
... WRITE THESE DISSOCIATED except concentrated sulfuric, it really is 97% H2SO4 and 3% water in the jug, so water is way outnumbered and the molecules don’t dissociate completely. If carbonic acid is formed as a product Æ CO2 + H2O. 5. Strong bases: hydroxides [and oxides] of IA and IIA* metals—write t ...
No Slide Title
... 2. Write the ionic equation showing the strong electrolytes completely dissociated into cations and anions. 3. Cancel the spectator ions on both sides of the ionic equation 4. Check that charges and number of atoms are balanced in the net ionic equation ...
... 2. Write the ionic equation showing the strong electrolytes completely dissociated into cations and anions. 3. Cancel the spectator ions on both sides of the ionic equation 4. Check that charges and number of atoms are balanced in the net ionic equation ...
anna-chrobok-silesian-university-of-technology
... - Diels-Alder reaction, - oxidation of alcohols and ketones. IONIC LIQUIDS as homogeneous and heterogeneous catalysts Recycling of ionic liquids prevents them from: - ending up in the aquatic environment, - release into the atmosphere (low volatility). ...
... - Diels-Alder reaction, - oxidation of alcohols and ketones. IONIC LIQUIDS as homogeneous and heterogeneous catalysts Recycling of ionic liquids prevents them from: - ending up in the aquatic environment, - release into the atmosphere (low volatility). ...
expected output
... Lectures and Tutorials SYLLABUS: Quadratic functions and equations. Surds, logarithms and indices. Permutations and combinations. Series; finite, infinite, arithmetic, geometric and binomial(positive integral index only)including applications to compound interest, approximartions, growth and decay. ...
... Lectures and Tutorials SYLLABUS: Quadratic functions and equations. Surds, logarithms and indices. Permutations and combinations. Series; finite, infinite, arithmetic, geometric and binomial(positive integral index only)including applications to compound interest, approximartions, growth and decay. ...
expected output
... Lectures and Tutorials SYLLABUS: Quadratic functions and equations. Surds, logarithms and indices. Permutations and combinations. Series; finite, infinite, arithmetic, geometric and binomial(positive integral index only)including applications to compound interest, approximartions, growth and decay. ...
... Lectures and Tutorials SYLLABUS: Quadratic functions and equations. Surds, logarithms and indices. Permutations and combinations. Series; finite, infinite, arithmetic, geometric and binomial(positive integral index only)including applications to compound interest, approximartions, growth and decay. ...
Spring 2014
... molecules omitted for clarity. Unshaded spheres represent hydrogen atoms or ions and gray spheres represent the anions. Which of the three is the strongest electrolyte? a) ...
... molecules omitted for clarity. Unshaded spheres represent hydrogen atoms or ions and gray spheres represent the anions. Which of the three is the strongest electrolyte? a) ...
Inorganic chemistry

Inorganic chemistry deals with the synthesis and behavior of inorganic and organometallic compounds. This field covers all chemical compounds except the myriad organic compounds (carbon based compounds, usually containing C-H bonds), which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, and there is much overlap, most importantly in the sub-discipline of organometallic chemistry. It has applications in every aspect of the chemical industry–including catalysis, materials science, pigments, surfactants, coatings, medicine, fuel, and agriculture.