Chemistry Midterm Review 2006
... These are topics from a traditional 1st quarter. If you used the thematic approach with me this year, you will notice that the topics are not in order of their presentation throughout the year. The final exam is cumulative so this review will help you with reviewing the concepts and calculations. No ...
... These are topics from a traditional 1st quarter. If you used the thematic approach with me this year, you will notice that the topics are not in order of their presentation throughout the year. The final exam is cumulative so this review will help you with reviewing the concepts and calculations. No ...
PAP Chemistry - Fall Final Review
... a. sodium-23 b. calcium-40 c. Cu d. Ag 10. How does mass number relate to number of protons when talking about isotopes? ...
... a. sodium-23 b. calcium-40 c. Cu d. Ag 10. How does mass number relate to number of protons when talking about isotopes? ...
Matter—anything that has mass and occupies space Weight—pull of
... Energy may be converted from one form to another Energy conversion is inefficient Some energy is “lost” as heat (partly unusable energy) ...
... Energy may be converted from one form to another Energy conversion is inefficient Some energy is “lost” as heat (partly unusable energy) ...
Arts and Sciences Program Chemistry Department Chemistry Placement Test
... (density of air = 1.29 g/dm3 at 25C). ...
... (density of air = 1.29 g/dm3 at 25C). ...
Document
... A molecular formula shows the exact number of atoms of each element in the individual molecules which are held together by weak attractive forces. An empirical formula shows the simplest whole-number ratio of the atoms in a substance ...
... A molecular formula shows the exact number of atoms of each element in the individual molecules which are held together by weak attractive forces. An empirical formula shows the simplest whole-number ratio of the atoms in a substance ...
Midterm Review
... Define the law of multiple proportions and provide examples of two compounds that illustrate the concept. ...
... Define the law of multiple proportions and provide examples of two compounds that illustrate the concept. ...
word-doc Practice for the final exam!
... c. compound d. pure substance e. solid 3. If matter is uniform throughout, cannot be separated into other substances by physical processes, but can be decomposed into other substances by chemical processes, it is ________. a. a heterogeneous mixture b. an element c. a homogeneous mixture d. a compou ...
... c. compound d. pure substance e. solid 3. If matter is uniform throughout, cannot be separated into other substances by physical processes, but can be decomposed into other substances by chemical processes, it is ________. a. a heterogeneous mixture b. an element c. a homogeneous mixture d. a compou ...
200 Ways to Pass the Chemistry - Home 15-16
... q = heat absorbed or released (Joules) m = mass of substance in grams c = specific heat capacity of substance (J/gC) … for water it’s 4.18 J/g C. t = temperature change in degrees Celsius What is the total number of joules of heat energy absorbed by 12 grams of water when it is heated from 30°C to ...
... q = heat absorbed or released (Joules) m = mass of substance in grams c = specific heat capacity of substance (J/gC) … for water it’s 4.18 J/g C. t = temperature change in degrees Celsius What is the total number of joules of heat energy absorbed by 12 grams of water when it is heated from 30°C to ...
Atoms, Molecules and Ions
... being the result of microscopic collisions of atoms (water molecules). ...
... being the result of microscopic collisions of atoms (water molecules). ...
CVB101 – Lecture 3 Chemical Bonding • Chemical bonding
... Common redox reactions Combination reactions – a reaction in which 2 or more substances combine to form a single product Decomposition reactions – the breakdown of a compound into 2 or more products ...
... Common redox reactions Combination reactions – a reaction in which 2 or more substances combine to form a single product Decomposition reactions – the breakdown of a compound into 2 or more products ...
3. atomic structure
... energy level to a higher energy level. When an electron returns from a higher energy state to a lower energy state, it emits a specific amount of energy usually in the form of light. This is known as a bright line spectrum, and can be used to identify an element like a fingerprint. ...
... energy level to a higher energy level. When an electron returns from a higher energy state to a lower energy state, it emits a specific amount of energy usually in the form of light. This is known as a bright line spectrum, and can be used to identify an element like a fingerprint. ...
Unit 2: Biochem Notes
... - A solution with a pH __________ 7, has more OH- ions than H+ ions, and is basic. - A solution with a pH _________ 7, has more H+ ions than OH- ions, and is acidic. b. buffer – Weak acids or bases that can react with strong acids or bases to prevent sharp, sudden changes in pH. Buffers make acidic ...
... - A solution with a pH __________ 7, has more OH- ions than H+ ions, and is basic. - A solution with a pH _________ 7, has more H+ ions than OH- ions, and is acidic. b. buffer – Weak acids or bases that can react with strong acids or bases to prevent sharp, sudden changes in pH. Buffers make acidic ...
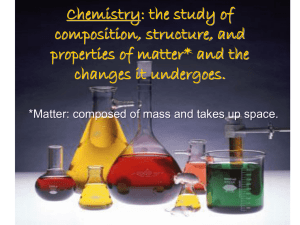
Chemistry: the study of composition, structure, and properties of
... An element is a pure substance made of only one kind of atom. – Carbon is an element made of only carbon atoms. Atoms of two or more elements bond together to make compounds. – CO2 (___ carbon atom and ___ oxygen atoms) – H2O2 (___ hydrogen atoms & ___ oxygen atoms) ...
... An element is a pure substance made of only one kind of atom. – Carbon is an element made of only carbon atoms. Atoms of two or more elements bond together to make compounds. – CO2 (___ carbon atom and ___ oxygen atoms) – H2O2 (___ hydrogen atoms & ___ oxygen atoms) ...
Name - Madison County Schools
... 17) If you get injured in the laboratory (cut, burn, etc.), what should you do? Immediately tell the instructor 18) Why is it important to check glassware for chips or cracks? Cracks and chips weaken glass so it more easily breaks 19) Is it appropriate to return all unused chemicals to their origin ...
... 17) If you get injured in the laboratory (cut, burn, etc.), what should you do? Immediately tell the instructor 18) Why is it important to check glassware for chips or cracks? Cracks and chips weaken glass so it more easily breaks 19) Is it appropriate to return all unused chemicals to their origin ...
Chapter 2 power point
... Filtration: Separates components of a mixture based upon differences in particle size. Filtration usually involves separating a precipitate from solution. Crystallization: Separation is based upon differences in solubility of the components in a mixture. Distillation: Separation is based upon differ ...
... Filtration: Separates components of a mixture based upon differences in particle size. Filtration usually involves separating a precipitate from solution. Crystallization: Separation is based upon differences in solubility of the components in a mixture. Distillation: Separation is based upon differ ...
Chapter 2 PPT - Richsingiser.com
... empirical formula that uses the smallest whole number subscripts to express the relative numbers of ions. • The relative numbers of ions in the empirical formula balances the charges to zero. • The formula of sodium chloride is NaCl, because the 1+ ions have to be present in a 1:1 ...
... empirical formula that uses the smallest whole number subscripts to express the relative numbers of ions. • The relative numbers of ions in the empirical formula balances the charges to zero. • The formula of sodium chloride is NaCl, because the 1+ ions have to be present in a 1:1 ...
Zinc isotopes in biology Oral tracers of enriched Zn and
... the atomic nucleus. A pure chemical substance composed of atoms with the same number of protons in the atomic nucleus [703]. gamma rays (gamma radiation) – a stream of high-energy electromagnetic radiation given off by an atomic nucleus undergoing radioactive decay. The energies of gamma rays are hi ...
... the atomic nucleus. A pure chemical substance composed of atoms with the same number of protons in the atomic nucleus [703]. gamma rays (gamma radiation) – a stream of high-energy electromagnetic radiation given off by an atomic nucleus undergoing radioactive decay. The energies of gamma rays are hi ...
Chemistry I – Fall 2004
... 38. In the laboratory, never dip a stirring rod into a reagent bottle because (A) the bottle may tip. (B) the rod might break. (C) the rod may puncture the bottle. (D) the contents of the bottle may become contaminated. (E) the amount of liquid remaining on the rod is too small to be used. 39. What ...
... 38. In the laboratory, never dip a stirring rod into a reagent bottle because (A) the bottle may tip. (B) the rod might break. (C) the rod may puncture the bottle. (D) the contents of the bottle may become contaminated. (E) the amount of liquid remaining on the rod is too small to be used. 39. What ...
Things to Know to Pass the Chemistry Regents
... 5. Orbitals: most probable location of electrons in e- cloud, modern (wave-mechanical) model 6. Mass number: protons + neutrons, C-14 has a mass of 14 (6p + 8n = 14) 7. Atomic number: equals # of protons, identifies element/atom *all atoms with 6p are carbon, all atoms of carbon have 6p 8. Number of ...
... 5. Orbitals: most probable location of electrons in e- cloud, modern (wave-mechanical) model 6. Mass number: protons + neutrons, C-14 has a mass of 14 (6p + 8n = 14) 7. Atomic number: equals # of protons, identifies element/atom *all atoms with 6p are carbon, all atoms of carbon have 6p 8. Number of ...
Name ………………………………………………… Unit 7: States of
... Crude oil is a mixture of many hydrocarbons that have different numbers of carbon atoms. The use of a fractionating tower allows the separation of this mixture based on the boiling points of the hydrocarbons. To begin the separation process, the crude oil is heated to about 400°C in a furnace, caus ...
... Crude oil is a mixture of many hydrocarbons that have different numbers of carbon atoms. The use of a fractionating tower allows the separation of this mixture based on the boiling points of the hydrocarbons. To begin the separation process, the crude oil is heated to about 400°C in a furnace, caus ...
Chapter 2 1
... thoughts in the form a table that recognized the periodic nature of the chemical properties known at the time. He also recognized that there were elements that had not been discovered and left blanks in his table where these elements would occur while predicting their properties. For this reason, th ...
... thoughts in the form a table that recognized the periodic nature of the chemical properties known at the time. He also recognized that there were elements that had not been discovered and left blanks in his table where these elements would occur while predicting their properties. For this reason, th ...
final exam practice test - Clayton State University
... It ignites and burns with a yellow flame when heated strongly. V. It is usually in the form of small crystals although it can occur as a powder. a.) I, II, IV d.) I and V ...
... It ignites and burns with a yellow flame when heated strongly. V. It is usually in the form of small crystals although it can occur as a powder. a.) I, II, IV d.) I and V ...
Nature of Atoms Atomic Structure Atomic number Atomic mass
... Inert (nonreactive) elements have all eight electrons Octet rule – atoms tend to establish completely full outer energy levels ...
... Inert (nonreactive) elements have all eight electrons Octet rule – atoms tend to establish completely full outer energy levels ...
Chapter 2: Atoms, Molecules, and Ions
... A) Each atom of oxygen is 16 times more massive than an atom of hydrogen. B) A given compound always contains exactly the same proportion of elements by mass. C) When two elements form a series of compounds, the ratios of masses that combine with 1 gram of the first element can always be reduced to ...
... A) Each atom of oxygen is 16 times more massive than an atom of hydrogen. B) A given compound always contains exactly the same proportion of elements by mass. C) When two elements form a series of compounds, the ratios of masses that combine with 1 gram of the first element can always be reduced to ...
Document
... 15) Name any two materials that can be classified as substances and give two ways that they are different from each other. Answers may vary 16) A combination of sugar and water would be classified as a: a) pure substance ...
... 15) Name any two materials that can be classified as substances and give two ways that they are different from each other. Answers may vary 16) A combination of sugar and water would be classified as a: a) pure substance ...