Ch. 1 Introduction: Matter and Measurement
... Baking a cake Adding a Crystal Light© packet to water Leaves on a tree turning red or yellow ...
... Baking a cake Adding a Crystal Light© packet to water Leaves on a tree turning red or yellow ...
Chapter 1 - TamAPChemistryHart
... Chapter 1 “Introduction: Matter and Measurement” Assignments Classification and Properties of Matter: Exercises: p.31: #11,15,16 11. Classify each of the following as a pure substance or a mixture; if a mixture, indicate whether it is homogeneous or heterogeneous: a) rice pudding b) seawater c) magn ...
... Chapter 1 “Introduction: Matter and Measurement” Assignments Classification and Properties of Matter: Exercises: p.31: #11,15,16 11. Classify each of the following as a pure substance or a mixture; if a mixture, indicate whether it is homogeneous or heterogeneous: a) rice pudding b) seawater c) magn ...
SCSD Physical Science 9th - Shenandoah Community Schools
... o Elements are listed in order according to the number of protons (atomic number)(I,D,M) o Repeating patterns of physical and chemical properties identify families of elements with similar properties (I, D, M) o The periodic table is a consequence of the repeating pattern of outermost electrons and ...
... o Elements are listed in order according to the number of protons (atomic number)(I,D,M) o Repeating patterns of physical and chemical properties identify families of elements with similar properties (I, D, M) o The periodic table is a consequence of the repeating pattern of outermost electrons and ...
NAME GRADED: LET IT BEGIN!!! ____ / 30 pts DIRECTIONS: Use
... energy). A radioactive isotope can spontaneously transmute into (turn into) an isotope of another element when these changes to the nucleus, changes the number of protons! Nuclear fission is a nuclear reaction in which a heavy nucleus (such as uranium) splits into two or more, lighter nuclei. As thi ...
... energy). A radioactive isotope can spontaneously transmute into (turn into) an isotope of another element when these changes to the nucleus, changes the number of protons! Nuclear fission is a nuclear reaction in which a heavy nucleus (such as uranium) splits into two or more, lighter nuclei. As thi ...
E/F Physical Science
... 3. Is the following sentence true or false? The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction. 4. Circle the letter of the correct answer. According to the equation C + O2 → CO2, how many carbon atoms react with 14 molecules of oxygen to form 14 ...
... 3. Is the following sentence true or false? The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction. 4. Circle the letter of the correct answer. According to the equation C + O2 → CO2, how many carbon atoms react with 14 molecules of oxygen to form 14 ...
e c n i
... The activation energy is the energy needed by a system to initiate the reaction. It is the minimum energy needed for a specific chemical reaction to occur. Once achieved, the reaction continues until reactants are ...
... The activation energy is the energy needed by a system to initiate the reaction. It is the minimum energy needed for a specific chemical reaction to occur. Once achieved, the reaction continues until reactants are ...
Chemical Equations
... separates 2 or more reactants or products “yield”, separates reactants from products. indicates a reversible reaction solid state. Placed after the formula of a substance Alternative to (s) but used ONLY for a solid PRODUCT, not reactants indicates a liquid reactant or product indicates an aqueous s ...
... separates 2 or more reactants or products “yield”, separates reactants from products. indicates a reversible reaction solid state. Placed after the formula of a substance Alternative to (s) but used ONLY for a solid PRODUCT, not reactants indicates a liquid reactant or product indicates an aqueous s ...
o C
... Elements are the simplest pure substances. An element cannot be changed into simpler substances by any chemical process. ...
... Elements are the simplest pure substances. An element cannot be changed into simpler substances by any chemical process. ...
Physical Science
... another substance ie. Water evaporates into water vapor, a rock is broken into pieces It’s like printing a word in a different font, it’s the same word just looks different! ...
... another substance ie. Water evaporates into water vapor, a rock is broken into pieces It’s like printing a word in a different font, it’s the same word just looks different! ...
Lecture 3
... of chemical research in Germany is the lack of general, and at the same time thorough chemical knowledge; no small number of our professors of chemistry, with great harm to our science, are laboring under this lack. A consequence of this is the spread of the weed of the apparently scholarly and clev ...
... of chemical research in Germany is the lack of general, and at the same time thorough chemical knowledge; no small number of our professors of chemistry, with great harm to our science, are laboring under this lack. A consequence of this is the spread of the weed of the apparently scholarly and clev ...
Chemical equations must be balanced.
... This equation is not balanced. There is one C on each side of the equation, so C is balanced. However, on the left side, H has a subscript of 4, which means there are four hydrogen atoms. On the right side, H has a subscript of 2, which means there are two hydrogen atoms. Also, there are two oxygen ...
... This equation is not balanced. There is one C on each side of the equation, so C is balanced. However, on the left side, H has a subscript of 4, which means there are four hydrogen atoms. On the right side, H has a subscript of 2, which means there are two hydrogen atoms. Also, there are two oxygen ...
The Wizard Test Maker
... that of magnesium oxide? (A) The O2– is more negatively charged than the Cl– ion. (B) The Cl– ion is larger than the O2– ion. (C) The Mg2+ is more positively charged than the Na+ ion. (D) Choices A and C are correct. (E) Choices B and C are correct. 125. What is main reason the boiling point of meth ...
... that of magnesium oxide? (A) The O2– is more negatively charged than the Cl– ion. (B) The Cl– ion is larger than the O2– ion. (C) The Mg2+ is more positively charged than the Na+ ion. (D) Choices A and C are correct. (E) Choices B and C are correct. 125. What is main reason the boiling point of meth ...
Class 11 Class 12 The p- Block Element • Group13 (B to Tl
... The first ionization energies of group 13 elements are less than the corresponding members of the alkaline earths. The sharp decrease in I.E. from B to Al is due to increase in size. In case of Ga, there are ten delectrons in its inner electronic configuration. The very high value of 3rd I. E. of th ...
... The first ionization energies of group 13 elements are less than the corresponding members of the alkaline earths. The sharp decrease in I.E. from B to Al is due to increase in size. In case of Ga, there are ten delectrons in its inner electronic configuration. The very high value of 3rd I. E. of th ...
1 Packet #3 Mass Relationships in Chemical Reactions How is
... 6. A sample of a liquid consisting of only C, H, and O and having a mass of 0.5438 g was burned in pure oxygen. The following products were obtained: 1.039 g CO2 and 0.6369 g H2O. What is the empirical formula of the compound? ...
... 6. A sample of a liquid consisting of only C, H, and O and having a mass of 0.5438 g was burned in pure oxygen. The following products were obtained: 1.039 g CO2 and 0.6369 g H2O. What is the empirical formula of the compound? ...
Matter and Energy Notes
... particles vibrate but can’t move around fixed shape fixed volume ...
... particles vibrate but can’t move around fixed shape fixed volume ...
Chapter 3
... colorless gas Oxygen, a diatomic and colorless gas Water, a clear liquid which is essential for most life forms ...
... colorless gas Oxygen, a diatomic and colorless gas Water, a clear liquid which is essential for most life forms ...
Chemistry SOL Review
... • To determine whether a bond is polar, nonpolar, or ionic, you must use a table of electronegativities. (This will be given to you on the SOL if you are supposed to use it.) When you subtract the two values, if the difference is… – …between 0 and 0.4, the bond is nonpolar, meaning the electrons are ...
... • To determine whether a bond is polar, nonpolar, or ionic, you must use a table of electronegativities. (This will be given to you on the SOL if you are supposed to use it.) When you subtract the two values, if the difference is… – …between 0 and 0.4, the bond is nonpolar, meaning the electrons are ...
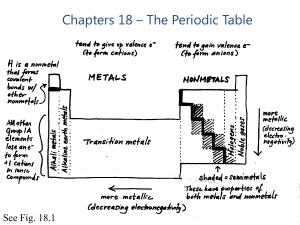
Chapters 18 – The Periodic Table
... trisulfide (P4S3), glass and binder. The phosphorus sulfide is easily ignited, the potassium chlorate decomposes to give oxygen, which in turn causes the phosphorus sulfide to burn more vigorously. The head of safety matches are made of an oxidizing agent such as potassium chlorate, mixed with sulfu ...
... trisulfide (P4S3), glass and binder. The phosphorus sulfide is easily ignited, the potassium chlorate decomposes to give oxygen, which in turn causes the phosphorus sulfide to burn more vigorously. The head of safety matches are made of an oxidizing agent such as potassium chlorate, mixed with sulfu ...
Unit 2.2 Test Review Key
... coefficients mean? How can you tell which elements are present. Know the Law of Conservation of Mass and how it is applied to chemical reactions Know what makes a compound organic or not Know the difference between physical and chemical properties and changes Exothermic and endothermic reactions Kno ...
... coefficients mean? How can you tell which elements are present. Know the Law of Conservation of Mass and how it is applied to chemical reactions Know what makes a compound organic or not Know the difference between physical and chemical properties and changes Exothermic and endothermic reactions Kno ...
ChemChpt 10 2014
... elements on both sides of the arrow, because mass can not be lost or gained in a reaction • don’t confuse subscripts with coefficients • subscripts determine the substance, coefficients tell us how much of that substance we have • we don’t change the subscripts, or we change the substance, rather we ...
... elements on both sides of the arrow, because mass can not be lost or gained in a reaction • don’t confuse subscripts with coefficients • subscripts determine the substance, coefficients tell us how much of that substance we have • we don’t change the subscripts, or we change the substance, rather we ...
The structure of Matter
... elements that are chemically combined. O The forces that hold atoms or ions together in a compound are called chemical bonds. O When a chemical reaction takes place, bonds are broken or formed, and a new substance with new properties is produced. O Atoms are rearranged! ...
... elements that are chemically combined. O The forces that hold atoms or ions together in a compound are called chemical bonds. O When a chemical reaction takes place, bonds are broken or formed, and a new substance with new properties is produced. O Atoms are rearranged! ...
How to Balance Chemical Equations
... In a chemical reaction atoms are rearranged as old chemical bonds are broken and new chemical bonds are formed. The 'law of conservation of __________' is supported as the weight doesn’t change between the mass of the reactants and the mass of the products. 2. How to balance a chemical equation In b ...
... In a chemical reaction atoms are rearranged as old chemical bonds are broken and new chemical bonds are formed. The 'law of conservation of __________' is supported as the weight doesn’t change between the mass of the reactants and the mass of the products. 2. How to balance a chemical equation In b ...
Chapter 1 Introduction to Chemistry
... of a mixture on the basis of the tendency of each to travel or be drawn across the surface of another material ...
... of a mixture on the basis of the tendency of each to travel or be drawn across the surface of another material ...