IGCSE SoW 2013
... Describe the reactions with oxygen in air of magnesium, carbon and sulphur, and the acidbase character of the oxides produced and link acid - base character of the oxides to the position of the element in the Periodic Table ...
... Describe the reactions with oxygen in air of magnesium, carbon and sulphur, and the acidbase character of the oxides produced and link acid - base character of the oxides to the position of the element in the Periodic Table ...
Extended Abstract
... Geology was always of interest to Lavoisier, whose first chemical study involved examination of gypsum. He copied nature by using water to examine the substance’s solubility and chemical properties. He wrote (Gurlac 1973; Lavoisier 1864-1893, p. 112), “Water, this almost universal solvent . . . is ...
... Geology was always of interest to Lavoisier, whose first chemical study involved examination of gypsum. He copied nature by using water to examine the substance’s solubility and chemical properties. He wrote (Gurlac 1973; Lavoisier 1864-1893, p. 112), “Water, this almost universal solvent . . . is ...
Review Packet - Newton.k12.ma.us
... with than if you use grams or pounds. Also, you can compare two quantities of moles to each other, but you cannot compare grams and pounds. 7. Hydrates are compounds formed by the union of water with some other substance, generally forming a neutral body, as certain crystallized salts. 8. The concen ...
... with than if you use grams or pounds. Also, you can compare two quantities of moles to each other, but you cannot compare grams and pounds. 7. Hydrates are compounds formed by the union of water with some other substance, generally forming a neutral body, as certain crystallized salts. 8. The concen ...
Chemical equilibrium and the kinetic theory of gases
... that they use in reactors. Knowledge of how these gases behave under different conditions of temperature and pressure is clearly going to be very important to a chemical engineer – and fortunately the behaviour of all gases is governed to a large extent by an equation known as the ideal gas law. You ...
... that they use in reactors. Knowledge of how these gases behave under different conditions of temperature and pressure is clearly going to be very important to a chemical engineer – and fortunately the behaviour of all gases is governed to a large extent by an equation known as the ideal gas law. You ...
eBook AQA GCSE Chemistry Unit C2 Part 1
... We know how to manipulate structures to make substances with perfect properties for particular purposes. Today, scientists are excited about nanoscience – the study of structures measuring around one billionth of a metre. Will discoveries at the nanoscale lead to new cancer cures, greener energy tec ...
... We know how to manipulate structures to make substances with perfect properties for particular purposes. Today, scientists are excited about nanoscience – the study of structures measuring around one billionth of a metre. Will discoveries at the nanoscale lead to new cancer cures, greener energy tec ...
Chemistry - Onslow College
... Merit requires you to provide reasons for how and why (in-depth understanding). Excellence requires you to show understanding as to how or why something occurs by linking chemistry ideas/principles (comprehensive understanding). It may involve you in justifying, relating, evaluating, comparing and c ...
... Merit requires you to provide reasons for how and why (in-depth understanding). Excellence requires you to show understanding as to how or why something occurs by linking chemistry ideas/principles (comprehensive understanding). It may involve you in justifying, relating, evaluating, comparing and c ...
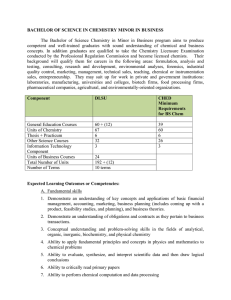
BACHELOR OF SCIENCE IN CHEMISTRY MINOR IN BUSINESS
... instrumentation in analytical and synthetic work, in relation to both organic and inorganic systems 4. Skills in the monitoring, by observation and measurement, of chemical properties, events, or changes, and the systematic and reliable recording and documentation thereof 5. Ability to evaluate and ...
... instrumentation in analytical and synthetic work, in relation to both organic and inorganic systems 4. Skills in the monitoring, by observation and measurement, of chemical properties, events, or changes, and the systematic and reliable recording and documentation thereof 5. Ability to evaluate and ...
1 Course Code– CH1141 Semester – I Credit
... Answer any 8 questions from the following. Each question carries two marks 11. State and explain Pauli’s exclusion principle. 12. Name two types of hydrogen bonding with example. 13. State and explain Fajan’s rule. 14. Define (i) work function (ii) Gibb’s free energy function. 15. State and explain ...
... Answer any 8 questions from the following. Each question carries two marks 11. State and explain Pauli’s exclusion principle. 12. Name two types of hydrogen bonding with example. 13. State and explain Fajan’s rule. 14. Define (i) work function (ii) Gibb’s free energy function. 15. State and explain ...
Chemistry Fall 2014 Review
... ____ 101. If some measurements agree closely with each other but differ widely from the actual value, these measurements are a. both accurate and precise. b. neither precise nor accurate. c. precise but not accurate. d. accurate but not precise. ____ 102. In which of the following measurements are ...
... ____ 101. If some measurements agree closely with each other but differ widely from the actual value, these measurements are a. both accurate and precise. b. neither precise nor accurate. c. precise but not accurate. d. accurate but not precise. ____ 102. In which of the following measurements are ...
The Chemistry of Burgers
... meat patty wasn’t exposed to the high temperature of the pan and so it wasn’t able to undergo the hightemperature Maillard reaction, which create these nice crusty brown bits that are very flavorful to us. KIRSHENBAUM: The Maillard reactions result in development of rich brown colors. And they also ...
... meat patty wasn’t exposed to the high temperature of the pan and so it wasn’t able to undergo the hightemperature Maillard reaction, which create these nice crusty brown bits that are very flavorful to us. KIRSHENBAUM: The Maillard reactions result in development of rich brown colors. And they also ...
Chemistry - College Catalog
... 20100-20200. Inorganic Chemistry I, II. PQ for 20100: CHEM 1110011200-11300 or equivalent, 22000 and 22100, or concurrent enrollment in 22100 or equivalent. PQ for 20200: CHEM 20100 and 22200. The extraordinarily diverse chemistry of the elements is organized in terms of molecular structure, electro ...
... 20100-20200. Inorganic Chemistry I, II. PQ for 20100: CHEM 1110011200-11300 or equivalent, 22000 and 22100, or concurrent enrollment in 22100 or equivalent. PQ for 20200: CHEM 20100 and 22200. The extraordinarily diverse chemistry of the elements is organized in terms of molecular structure, electro ...
atoms. - Unicam
... the basis of the modern theory. However, Dalton postulated the wrong assumption that atoms were not possible to divide ...
... the basis of the modern theory. However, Dalton postulated the wrong assumption that atoms were not possible to divide ...
9/21 properties of matter ppt
... However, when you have a liquid/liquid mixture, the mixture must be separated by a more complicated technique called fractional distillation. When the mixture of liquids is heated, the liquid with the lowest boiling point is distilled first. This liquid turns into a vapor (gas) and flows out the dis ...
... However, when you have a liquid/liquid mixture, the mixture must be separated by a more complicated technique called fractional distillation. When the mixture of liquids is heated, the liquid with the lowest boiling point is distilled first. This liquid turns into a vapor (gas) and flows out the dis ...
Chemical Reactions
... A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances – The reactant in a decomposition reaction must be a compound – the products may be elements or compounds AB → A + B ...
... A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances – The reactant in a decomposition reaction must be a compound – the products may be elements or compounds AB → A + B ...
The Atomic Theory Chem 111
... 1 -Law of Definite Proportions - a compound is composed of two or more elements chemically combined in a definite ratio by weight. 2 - Law of Multiple Proportions: when any two elements (A+B) combine to form more than one compound, the different weights of B that combine with A have a small whole nu ...
... 1 -Law of Definite Proportions - a compound is composed of two or more elements chemically combined in a definite ratio by weight. 2 - Law of Multiple Proportions: when any two elements (A+B) combine to form more than one compound, the different weights of B that combine with A have a small whole nu ...
English Medium
... 2. Explain why dogs pant during hot summer days using the concept of evaporation? A. Dogs do not have sweat pores on their skin, but only at their feet. The sweat produced by evaporation would not have way to go out. Hence it pant during hot summer days to maintain its body temperature balance. 3. C ...
... 2. Explain why dogs pant during hot summer days using the concept of evaporation? A. Dogs do not have sweat pores on their skin, but only at their feet. The sweat produced by evaporation would not have way to go out. Hence it pant during hot summer days to maintain its body temperature balance. 3. C ...
Carbene Singlets, Triplets, and the Physics that
... while knowing a set of rules for writing them allows them to be consistent with experiments in cases where there are no extremely similar energy levels in the interaction diagram, they are not suitable for discerning numerical results. However, as will be shown in more detail later, these molecular ...
... while knowing a set of rules for writing them allows them to be consistent with experiments in cases where there are no extremely similar energy levels in the interaction diagram, they are not suitable for discerning numerical results. However, as will be shown in more detail later, these molecular ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.