17 ADSORPTION AND CATALYSIS S MODULE - 5
... S urface of solids plays a crucial role in many physical and chemical phenomena. There are two main reasons for this special role. Firstly, the surface of a substance interacts first with its surroundings. Secondly, the surface molecules are in a different state as compared to the molecules in the i ...
... S urface of solids plays a crucial role in many physical and chemical phenomena. There are two main reasons for this special role. Firstly, the surface of a substance interacts first with its surroundings. Secondly, the surface molecules are in a different state as compared to the molecules in the i ...
7.1 Describing Reactions
... reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen that reacted. ...
... reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen that reacted. ...
Slide 1
... reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen that reacted. ...
... reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen that reacted. ...
7.1 Describing Reactions
... reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen that reacted. ...
... reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen that reacted. ...
Basic Agricultural Chemistry - Macmillan Education South Africa
... group share similar chemical properties. The Roman numerals listed above each group are the usual number of valence electrons. For example: l Group IA is the alkali metals where the element has one electron in the orbital, for example sodium, potassium and rubidium. Alkali metals form salts and man ...
... group share similar chemical properties. The Roman numerals listed above each group are the usual number of valence electrons. For example: l Group IA is the alkali metals where the element has one electron in the orbital, for example sodium, potassium and rubidium. Alkali metals form salts and man ...
Lone pairs
... Form pairs of two….quickly and quietly. Switch desks so that pairs are sitting next to each ...
... Form pairs of two….quickly and quietly. Switch desks so that pairs are sitting next to each ...
+2 - Fort Thomas Independent Schools
... 1. Write formulas for the binary ionic compounds formed between the following elements: a. potassium and iodine b. magnesium and chlorine c. sodium and sulfur d. aluminum and sulfur e. aluminum and nitrogen 2. Name the binary ionic compounds indicated by the following formulas: a. AgCl ...
... 1. Write formulas for the binary ionic compounds formed between the following elements: a. potassium and iodine b. magnesium and chlorine c. sodium and sulfur d. aluminum and sulfur e. aluminum and nitrogen 2. Name the binary ionic compounds indicated by the following formulas: a. AgCl ...
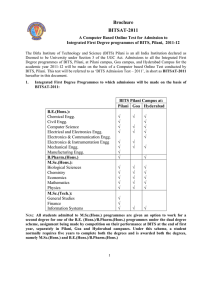
Brochure BITSAT-2011
... If a candidate answers all the 150 questions (without skipping any question), the candidate will have an option of attempting 12 (twelve) extra questions, if there is still time left. These extra questions will be from Physics, Chemistry, and Mathematics only; four questions from each part. Further, ...
... If a candidate answers all the 150 questions (without skipping any question), the candidate will have an option of attempting 12 (twelve) extra questions, if there is still time left. These extra questions will be from Physics, Chemistry, and Mathematics only; four questions from each part. Further, ...
chem equation Pkt Student2
... c) Aluminum sulfate reacts with calcium hydroxide to form aluminum hydroxide and calcium sulfate. ...
... c) Aluminum sulfate reacts with calcium hydroxide to form aluminum hydroxide and calcium sulfate. ...
SUP1111 11 - The Open University
... various _____________ molecules. In _______________ organisms (such as bacteria) the circular molecules that carry the genetic information lie naked in the ____________. Bacteria and viruses which infect humans are known as ____________. The immune system produces ________________, which try and eli ...
... various _____________ molecules. In _______________ organisms (such as bacteria) the circular molecules that carry the genetic information lie naked in the ____________. Bacteria and viruses which infect humans are known as ____________. The immune system produces ________________, which try and eli ...
Contents
... wastage with some of one reactant being left over. In many processes, in addition to the required product, some waste chemicals are produced. These not only contribute to pollution problems but they are also costly for the chemical company. For this reason, chemists are devising new chemical reactio ...
... wastage with some of one reactant being left over. In many processes, in addition to the required product, some waste chemicals are produced. These not only contribute to pollution problems but they are also costly for the chemical company. For this reason, chemists are devising new chemical reactio ...
Sec. 10.3 - Midland Park School District
... volumes of gases at the same temperature and pressure contain equal numbers of particles. Further, one mole (or 6.02 x 1023 particles) of a gas (no matter what it is) will always have the same volume. ...
... volumes of gases at the same temperature and pressure contain equal numbers of particles. Further, one mole (or 6.02 x 1023 particles) of a gas (no matter what it is) will always have the same volume. ...
9647 H2 Chemistry
... Candidates will be assumed to have knowledge and understanding of Chemistry at O level, as a single subject or as part of a balanced science course. This syllabus is designed to place less emphasis on factual material and greater emphasis on the understanding and application of scientific concepts a ...
... Candidates will be assumed to have knowledge and understanding of Chemistry at O level, as a single subject or as part of a balanced science course. This syllabus is designed to place less emphasis on factual material and greater emphasis on the understanding and application of scientific concepts a ...
Syllabus - Chemistry
... component would be evaluated in the same manner as project works are evaluated. ...
... component would be evaluated in the same manner as project works are evaluated. ...
Compounds
... 43. Metals conduct electricity when solid while ionic compounds only conduct electricity when molten or aqueous. Explain this difference in properties. 44. Distinguish homogenous alloys from heterogeneous alloys. 45. Sketch a homogeneous interstitial alloy. How would this differ from an intermetalli ...
... 43. Metals conduct electricity when solid while ionic compounds only conduct electricity when molten or aqueous. Explain this difference in properties. 44. Distinguish homogenous alloys from heterogeneous alloys. 45. Sketch a homogeneous interstitial alloy. How would this differ from an intermetalli ...
chap-4-atomic-weights
... Dalton's atomic theory predicted the impossibility of many things. For example, it should be impossible to make water from gold because gold doesn't contain the hydrogen and oxygen atoms needed to make water. However, Dalton's theory could not predict: -What is actually possible? Given that water an ...
... Dalton's atomic theory predicted the impossibility of many things. For example, it should be impossible to make water from gold because gold doesn't contain the hydrogen and oxygen atoms needed to make water. However, Dalton's theory could not predict: -What is actually possible? Given that water an ...
lecture ch1-3 chem161pikul
... Mental picture that explains observed laws Tentative explanation of data Make predictions Leads to further tests Go to laboratory and perform experiments ...
... Mental picture that explains observed laws Tentative explanation of data Make predictions Leads to further tests Go to laboratory and perform experiments ...
2 - TEST BANK 360
... atoms being the same (oxygen). Since each "ball" represents an individual atom, the three models on the left can be eliminated since they don't contain the correct number of atoms. Keeping in mind that balls of the same color represent the same element, only the model on the far right contains two e ...
... atoms being the same (oxygen). Since each "ball" represents an individual atom, the three models on the left can be eliminated since they don't contain the correct number of atoms. Keeping in mind that balls of the same color represent the same element, only the model on the far right contains two e ...
Section 8.3 Names and Formulas of Ionic Compounds Formula Unit
... Compounds that contain ionic bonds are called ionic compounds. • Ionic bonds that occur between metals and the non metal oxygen are called ...
... Compounds that contain ionic bonds are called ionic compounds. • Ionic bonds that occur between metals and the non metal oxygen are called ...
Document
... Liquids have intermediate properties between solids and gases. Liquids are almost incompressible, have definite volume and assume the shape of the container. Densities of liquids are usually lower than that of their solids. Water is an exception. ...
... Liquids have intermediate properties between solids and gases. Liquids are almost incompressible, have definite volume and assume the shape of the container. Densities of liquids are usually lower than that of their solids. Water is an exception. ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.