AS Chemistry - Crawshaw Academy
... and must be returned at the end of each academic year. Should a student leave the course for any reason agreed with Mrs Pheasey the textbook must be returned or paid for. Chemistry staff @ Crawshaw – Students will be taught by both Mrs Dhesi Assistant Headteacher and Miss Dale Head of Science ...
... and must be returned at the end of each academic year. Should a student leave the course for any reason agreed with Mrs Pheasey the textbook must be returned or paid for. Chemistry staff @ Crawshaw – Students will be taught by both Mrs Dhesi Assistant Headteacher and Miss Dale Head of Science ...
Chapters 14
... 7. What is the molarity of a solution made by dissolving 9.1 g of H3PO4 in enough water to make 22.3 L of solution? Assume that H3PO4 ionizes completely in water to H+ and PO43ions. What is the pH of the solution? Find the concentration of OH-? ...
... 7. What is the molarity of a solution made by dissolving 9.1 g of H3PO4 in enough water to make 22.3 L of solution? Assume that H3PO4 ionizes completely in water to H+ and PO43ions. What is the pH of the solution? Find the concentration of OH-? ...
Year End Chemistry Review
... 10. Fission vs Fusion. Describe each process and explain how atoms were made. 11. Place the following numbers into or take them out of scientific notation: a) 3,000,000 b) 321,000 c) 0.00000000248 d)74.3 e) 7.419 x 104 f) 9.16 x 10-8 12. How many valence electrons are in each of the following elemen ...
... 10. Fission vs Fusion. Describe each process and explain how atoms were made. 11. Place the following numbers into or take them out of scientific notation: a) 3,000,000 b) 321,000 c) 0.00000000248 d)74.3 e) 7.419 x 104 f) 9.16 x 10-8 12. How many valence electrons are in each of the following elemen ...
Physical and Chemical Changes Worksheet
... Part A Can you recognize the chemical and physical changes that happen all around us? If you change the way something looks, but haven’t made a new substance, a physical change (P) has occurred. If the substance has been changes into another substance, a chemical change (C) has occurred. ...
... Part A Can you recognize the chemical and physical changes that happen all around us? If you change the way something looks, but haven’t made a new substance, a physical change (P) has occurred. If the substance has been changes into another substance, a chemical change (C) has occurred. ...
Chemistry Standards and Frameworks
... 1. c.: Students know how to use the periodic table to identify alkali metals, alkaline earth metals and transition metals, trends in ionization energy, electro negativity, and the relative sizes of ions and atoms. A few other groups are given family names. These include the alkali metals (Group 1), ...
... 1. c.: Students know how to use the periodic table to identify alkali metals, alkaline earth metals and transition metals, trends in ionization energy, electro negativity, and the relative sizes of ions and atoms. A few other groups are given family names. These include the alkali metals (Group 1), ...
Review_WB_1
... ml, what will the new volume be? ____________ Draw a picture showing the new volume. ...
... ml, what will the new volume be? ____________ Draw a picture showing the new volume. ...
chemical reaction
... Chemical reactions are described by chemical equations. A chemical equation represents, with symbols and formulas, the identities and relative molecular or molar amounts of the reactants and products in a chemical reaction. For example, the following chemical equation shows that the reactant ammoni ...
... Chemical reactions are described by chemical equations. A chemical equation represents, with symbols and formulas, the identities and relative molecular or molar amounts of the reactants and products in a chemical reaction. For example, the following chemical equation shows that the reactant ammoni ...
CHEM 400 - El Camino College
... to use any electronic devices except for your own electronic calculator. You cannot share a calculator with a classmate or use your cell phone in place of a calculator. Your cell phone cannot be in your hands at any time during the exam. It must be kept in your pocket or in your bag. In case you hav ...
... to use any electronic devices except for your own electronic calculator. You cannot share a calculator with a classmate or use your cell phone in place of a calculator. Your cell phone cannot be in your hands at any time during the exam. It must be kept in your pocket or in your bag. In case you hav ...
chapt 1 - Cantt Academy, Tahli Mohri Chowk, Rawalpindi
... Pseudo – Science or Alchemy:In the beginning of chemistry during 600 – 1600 AD some scientist tried to convert cheap metals in to gold. They performed many experiment but could not succeed and wasted their time and money. These scientists are called alchemists and this branch of chemistry is called ...
... Pseudo – Science or Alchemy:In the beginning of chemistry during 600 – 1600 AD some scientist tried to convert cheap metals in to gold. They performed many experiment but could not succeed and wasted their time and money. These scientists are called alchemists and this branch of chemistry is called ...
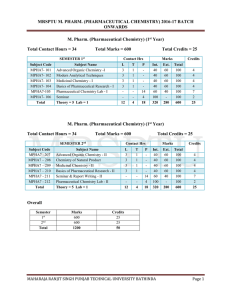
mrsptu m. pharm. (pharmaceutical chemistry) 2016
... John Wiley and Sons, New York. 2. L.G. Chatten, ‘Pharmaceutical Chemistry’, Vol. I & II, Marcel Dekker, New York. 3. W.D. James and H.T. Kenneth, ‘Analytical Chemistry by Open Learning: Thermal Methods. John Wiley and Sons, New York. 4. R.J. Abraham, J. Fisher and P. Bftus, ‘Introduction to NMR Spec ...
... John Wiley and Sons, New York. 2. L.G. Chatten, ‘Pharmaceutical Chemistry’, Vol. I & II, Marcel Dekker, New York. 3. W.D. James and H.T. Kenneth, ‘Analytical Chemistry by Open Learning: Thermal Methods. John Wiley and Sons, New York. 4. R.J. Abraham, J. Fisher and P. Bftus, ‘Introduction to NMR Spec ...
Unit 6 Worksheet Package
... between these two types of ions forms an _____________ bond. Nearly all ionic compounds are _____________ solids at room temperature. In these solids the total _____________ charge is balanced by the total _____________ charge. Ionic compounds in general have very _____________ melting points. This ...
... between these two types of ions forms an _____________ bond. Nearly all ionic compounds are _____________ solids at room temperature. In these solids the total _____________ charge is balanced by the total _____________ charge. Ionic compounds in general have very _____________ melting points. This ...
Chapter 1
... a) rice pudding Heterogeneous mixture b) seawater Homogeneous mixture unless there are undissolved particles such as sand, then heterogeneous c) magnesium Element d) gasoline Homogeneous mixture ...
... a) rice pudding Heterogeneous mixture b) seawater Homogeneous mixture unless there are undissolved particles such as sand, then heterogeneous c) magnesium Element d) gasoline Homogeneous mixture ...
4.1Atoms and Isotopes
... Carbon has three isotopes: C-12 (most abundant), C-13 (used in medical imagingMRI), and C-14 (used for dating fossils) Tin (Sn) has the most isotopes of any element at 10 Many isotopes are radioactive (unstable nucleus that will eventually break apart and release energy in sometimes harmful forms – ...
... Carbon has three isotopes: C-12 (most abundant), C-13 (used in medical imagingMRI), and C-14 (used for dating fossils) Tin (Sn) has the most isotopes of any element at 10 Many isotopes are radioactive (unstable nucleus that will eventually break apart and release energy in sometimes harmful forms – ...
Balancing Chemical Reactions
... Rules 1.) The formulas of the reactants and products cannot be changed, do not alter subscripts or charges. 2.) The only numbers that can be changed are the numbers indicating how many molecules or atoms, which are called coefficients. 3.) A coefficient is assumed to be one if there is not a number ...
... Rules 1.) The formulas of the reactants and products cannot be changed, do not alter subscripts or charges. 2.) The only numbers that can be changed are the numbers indicating how many molecules or atoms, which are called coefficients. 3.) A coefficient is assumed to be one if there is not a number ...
SAMPLE QUESTION PAPER-II Chemistry (Theory) Class-XII
... Neetu and Asha look organic compound synthesis as their chemistry project. They prepare benzene diazonium chloride and stored it at room temperature. Due to holiday, they start preparing azodye but it cannot be prepared. Then their friend Reena told them to prepare benzene diazonium chloride again a ...
... Neetu and Asha look organic compound synthesis as their chemistry project. They prepare benzene diazonium chloride and stored it at room temperature. Due to holiday, they start preparing azodye but it cannot be prepared. Then their friend Reena told them to prepare benzene diazonium chloride again a ...
Practice MSL Multiple Choice 1. Compared to the charge and mass
... 115. A solid is dissolved in a beaker of water. Which observation suggests that the process is endothermic? a. b. c. d. ...
... 115. A solid is dissolved in a beaker of water. Which observation suggests that the process is endothermic? a. b. c. d. ...
Downloads - Dr. Sahu`s Bio Classes, Best Coaching for NEET, PMT
... not have originated spontaneously from nonliving matter----------- meat was not spoiled, when heated and kept sealed in a vessel. Q.5. Swan-necked flask experiment was done by----- Louis Pasteur Q.6. Louis Pasteur is famous for ----------- Germ theory of disease Q.7. The idea that life originates fr ...
... not have originated spontaneously from nonliving matter----------- meat was not spoiled, when heated and kept sealed in a vessel. Q.5. Swan-necked flask experiment was done by----- Louis Pasteur Q.6. Louis Pasteur is famous for ----------- Germ theory of disease Q.7. The idea that life originates fr ...
advanced placement chemistry alamo heights high school scope
... This AP Chemistry course is designed to be the equivalent of the general chemistry course usually taken during the first year of college. For most students, the course enables them to undertake, as a ...
... This AP Chemistry course is designed to be the equivalent of the general chemistry course usually taken during the first year of college. For most students, the course enables them to undertake, as a ...
Chapter III: Matter - Norwell Public Schools
... As temperature and pressure change, substances change from one phase to another. ...
... As temperature and pressure change, substances change from one phase to another. ...
chemistry - billpalmer
... atoms 2) All atoms of the same element are identical; different atoms are different 3) Atoms cannot be subdivided, created, or destroyed 4) atoms combine in simple whole number ratios to form chemical compounds 5) In chemical reactions, atoms are combined, separated, or rearranged ...
... atoms 2) All atoms of the same element are identical; different atoms are different 3) Atoms cannot be subdivided, created, or destroyed 4) atoms combine in simple whole number ratios to form chemical compounds 5) In chemical reactions, atoms are combined, separated, or rearranged ...
History and Current Status of the Plastics Industry
... • Example, steam will take the shape of the container and occupy the entire volume of the container ...
... • Example, steam will take the shape of the container and occupy the entire volume of the container ...
File
... They can only be observed when matter goes through a chemical change, can’t know just by looking at it Examples: Flammability, Ability to react with specific materials ...
... They can only be observed when matter goes through a chemical change, can’t know just by looking at it Examples: Flammability, Ability to react with specific materials ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.