HW Notes: Nuclear Chemistry - Liberty Union High School District
... The alpha particle (a) is deflected to some extent toward the negative plate. This indicated that it is positively (+) charged and has a fairly large mass. Today we know that an a particle is the same as the nucleus of a He atom. ...
... The alpha particle (a) is deflected to some extent toward the negative plate. This indicated that it is positively (+) charged and has a fairly large mass. Today we know that an a particle is the same as the nucleus of a He atom. ...
A1990CG38700001
... Early measurements of electron Alt for different materials appeared to cluster about a common curve September 20, 1989 when plotted versus kinetic energy This curve became known as the “universal curve” and wasa useDuring the 1960s several types of instruments be- ful guide, even though there was no ...
... Early measurements of electron Alt for different materials appeared to cluster about a common curve September 20, 1989 when plotted versus kinetic energy This curve became known as the “universal curve” and wasa useDuring the 1960s several types of instruments be- ful guide, even though there was no ...
Electrical Properties
... Free Electron Theory Outermost electrons of the atoms take part in conduction These electrons are assumed to be free to move through the whole solid Free electron cloud / gas, Fermi gas Potential field due to ion-cores is assumed constant potential energy of electrons is not a function of ...
... Free Electron Theory Outermost electrons of the atoms take part in conduction These electrons are assumed to be free to move through the whole solid Free electron cloud / gas, Fermi gas Potential field due to ion-cores is assumed constant potential energy of electrons is not a function of ...
Radio waves belong to a family The
... Family Trait 5: Particle Behavior • The frequency of an EM wave is related to its energy by the formula f = E/h (more commonly written as E = h·f) h is Planck’s constant = 6.626 x 10-26 J/Hz ...
... Family Trait 5: Particle Behavior • The frequency of an EM wave is related to its energy by the formula f = E/h (more commonly written as E = h·f) h is Planck’s constant = 6.626 x 10-26 J/Hz ...
The Wave Nature of Light
... • A certain photon of light has a wavelength of 422 nm. What is the frequency of the light? • What is the energy of a quantum of light with a frequency of 7.39 x 1014 Hz? • A certain red light has a wavelength of 680 nm. What is the frequency of the light? • A certain blue light has a frequency of ...
... • A certain photon of light has a wavelength of 422 nm. What is the frequency of the light? • What is the energy of a quantum of light with a frequency of 7.39 x 1014 Hz? • A certain red light has a wavelength of 680 nm. What is the frequency of the light? • A certain blue light has a frequency of ...
Chapter 7(Hill/Petrucci/McCreary/Perry Introduction to Atomic
... two electrons may occupy any orbital, but must have opposed spins (+1/2 and -1/2) to minimize electron repulsion due to (-) charges on each electron Summary: Quantum Numbers and Electrons in Atoms each electron in a given atom has a unique set of four quantum numbers principal quantum number (n) … “ ...
... two electrons may occupy any orbital, but must have opposed spins (+1/2 and -1/2) to minimize electron repulsion due to (-) charges on each electron Summary: Quantum Numbers and Electrons in Atoms each electron in a given atom has a unique set of four quantum numbers principal quantum number (n) … “ ...
Pauli Exclusion Principle
... The aufbau principle is really a thought process in which we think about building up an atom from the one that preceeds it in atomic number, by adding a proton and neutrons to the nucleus and one electron to the appropriate atomic orbital. There are some exceptions to the to the aufbau principle. T ...
... The aufbau principle is really a thought process in which we think about building up an atom from the one that preceeds it in atomic number, by adding a proton and neutrons to the nucleus and one electron to the appropriate atomic orbital. There are some exceptions to the to the aufbau principle. T ...
The Many Faces of the Sun
... Flare radiation and emission mechanisms (contd.) γ-ray emission (contd.) - narrow lines in 4-7 MeV range produced when accelerated p+ and α particles interact with ambient heavy nuclei. - strongest γ-ray line is the neutron capture line at 2.23 MeV, with another strong line at 0.511 MeV due to posi ...
... Flare radiation and emission mechanisms (contd.) γ-ray emission (contd.) - narrow lines in 4-7 MeV range produced when accelerated p+ and α particles interact with ambient heavy nuclei. - strongest γ-ray line is the neutron capture line at 2.23 MeV, with another strong line at 0.511 MeV due to posi ...
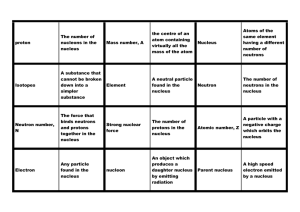
The Atoms Test Study Guide
... ________ if they have the correct amount of _____________. 5. The Modern Theory of the Atom says that you can never predict _____________________ and that they do not travel in particular paths around the nucleus but instead travel in ______________________. 6. Electrons that are closer to the nucle ...
... ________ if they have the correct amount of _____________. 5. The Modern Theory of the Atom says that you can never predict _____________________ and that they do not travel in particular paths around the nucleus but instead travel in ______________________. 6. Electrons that are closer to the nucle ...
Electrons as waves
... • It is impossible to determine simultaneously both the position, and velocity, of an electron or any other particle. ...
... • It is impossible to determine simultaneously both the position, and velocity, of an electron or any other particle. ...
The Bohr model for the electrons
... The Quantum Mechanics: waves of uncertainty System developed that incorporated these concepts and produced an orbital picture of the electrons No longer think of electrons as particles with precise location, but as waves which have probability of being in some region of the atom – the orbital Impos ...
... The Quantum Mechanics: waves of uncertainty System developed that incorporated these concepts and produced an orbital picture of the electrons No longer think of electrons as particles with precise location, but as waves which have probability of being in some region of the atom – the orbital Impos ...
photon particle - wave duality
... 1. The failure of classical physics to explain the spectrum of blackbody radiation (the “ultraviolet catastrophe”), and the successful explanation given by Planck based on a picture in which electromagnetic radiation is carried in particle-like bunches called “photons.” 2. The failure of classical p ...
... 1. The failure of classical physics to explain the spectrum of blackbody radiation (the “ultraviolet catastrophe”), and the successful explanation given by Planck based on a picture in which electromagnetic radiation is carried in particle-like bunches called “photons.” 2. The failure of classical p ...
2 Galactic radiation fields
... bound-free emission consists of a continuum (albeit not a particularly flat one), rather than a series of distinct spectral lines. • It is also worth noting that although the mean kinetic energy of the electrons in the plasma is simply 32 kT , the mean value of Ekin,e is slightly lower, because lowe ...
... bound-free emission consists of a continuum (albeit not a particularly flat one), rather than a series of distinct spectral lines. • It is also worth noting that although the mean kinetic energy of the electrons in the plasma is simply 32 kT , the mean value of Ekin,e is slightly lower, because lowe ...
CH 101 Study Guide Test 2
... Identify indicators of a chemical change Know the difference between a physical and chemical change (and examples) Identify compounds that are soluble or insoluble What happens when an ionic compound is added to water Balance a chemical equation Identify spectator ions Identify specific types of rea ...
... Identify indicators of a chemical change Know the difference between a physical and chemical change (and examples) Identify compounds that are soluble or insoluble What happens when an ionic compound is added to water Balance a chemical equation Identify spectator ions Identify specific types of rea ...
X-ray photoelectron spectroscopy - An introduction
... transmission function of the analyser. In principle the following equation can be used: I=JρσKλ I is the electron intensity J is the photon flux, ρ is the concentration of the atom or ion in the solid, σ s is the cross-section for photoelectron production (which depends on the element and en ...
... transmission function of the analyser. In principle the following equation can be used: I=JρσKλ I is the electron intensity J is the photon flux, ρ is the concentration of the atom or ion in the solid, σ s is the cross-section for photoelectron production (which depends on the element and en ...
Bremsstrahlung
Bremsstrahlung (German pronunciation: [ˈbʁɛmsˌʃtʁaːlʊŋ], from bremsen ""to brake"" and Strahlung ""radiation"", i.e. ""braking radiation"" or ""deceleration radiation"") is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon, thus satisfying the law of conservation of energy. The term is also used to refer to the process of producing the radiation. Bremsstrahlung has a continuous spectrum, which becomes more intense and whose peak intensity shifts toward higher frequencies as the change of the energy of the accelerated particles increases.Strictly speaking, braking radiation is any radiation due to the acceleration of a charged particle, which includes synchrotron radiation, cyclotron radiation, and the emission of electrons and positrons during beta decay. However, the term is frequently used in the more narrow sense of radiation from electrons (from whatever source) slowing in matter.Bremsstrahlung emitted from plasma is sometimes referred to as free/free radiation. This refers to the fact that the radiation in this case is created by charged particles that are free both before and after the deflection (acceleration) that caused the emission.