Calculating a substance`s gram formula mass
... it experimentally by using it to determine accurate atomic weights. Avogadro's number can be determined experimentally by observing the actual mass (usually via the momentum) of a "single" atom or molecule, then comparing that to the mass of one grammolecular-weight (or mole) of the same substance. ...
... it experimentally by using it to determine accurate atomic weights. Avogadro's number can be determined experimentally by observing the actual mass (usually via the momentum) of a "single" atom or molecule, then comparing that to the mass of one grammolecular-weight (or mole) of the same substance. ...
Chapter 6. Electronic Structure of Atoms
... • has integer values between –l and +l • are (2l+1) possible values of ml • example, for l = 1, there are (21+1) = 3 values of ml : 0, +1, and -1 • consequently, for l = 1, there are 3 orbitals: px, py and pz. • Collection of orbitals with same value of n called an electron shell • n2 orbitals in a ...
... • has integer values between –l and +l • are (2l+1) possible values of ml • example, for l = 1, there are (21+1) = 3 values of ml : 0, +1, and -1 • consequently, for l = 1, there are 3 orbitals: px, py and pz. • Collection of orbitals with same value of n called an electron shell • n2 orbitals in a ...
the pauli principle and the periodic table of the
... One way of systematizing the structure of the periodic table is to draw an “energy level” diagram for a hypothetical atom. The order of the levels tells the order of the filling of shells. Note that we do not have an actual level diagram for any actual atom and therfore we do not put any actual nume ...
... One way of systematizing the structure of the periodic table is to draw an “energy level” diagram for a hypothetical atom. The order of the levels tells the order of the filling of shells. Note that we do not have an actual level diagram for any actual atom and therfore we do not put any actual nume ...
Chemistry: Matter and Change
... • Compare the wave and particle natures of light. • Define a quantum of energy, and explain how it is related to an energy change of matter. • Contrast continuous electromagnetic spectra and atomic emission spectra. radiation: the rays and particles —alpha particles, beta particles, and gamma rays—t ...
... • Compare the wave and particle natures of light. • Define a quantum of energy, and explain how it is related to an energy change of matter. • Contrast continuous electromagnetic spectra and atomic emission spectra. radiation: the rays and particles —alpha particles, beta particles, and gamma rays—t ...
File
... • Compare the wave and particle natures of light. • Define a quantum of energy, and explain how it is related to an energy change of matter. • Contrast continuous electromagnetic spectra and atomic emission spectra. radiation: the rays and particles —alpha particles, beta particles, and gamma rays—t ...
... • Compare the wave and particle natures of light. • Define a quantum of energy, and explain how it is related to an energy change of matter. • Contrast continuous electromagnetic spectra and atomic emission spectra. radiation: the rays and particles —alpha particles, beta particles, and gamma rays—t ...
Laser - nptel
... The photons that are incident and those which are generated may be absorbed by atoms in ground states, leading to depletion in the number of photons. 2. Interaction of Radiation with Matter: In order to have an insight into the principle of laser, we need to understand the way radiation field intera ...
... The photons that are incident and those which are generated may be absorbed by atoms in ground states, leading to depletion in the number of photons. 2. Interaction of Radiation with Matter: In order to have an insight into the principle of laser, we need to understand the way radiation field intera ...
Chapter 3 Test Review
... and finish by Wednesday. - Homework for all of chapter one is due before break of else all days will be minimal. ...
... and finish by Wednesday. - Homework for all of chapter one is due before break of else all days will be minimal. ...
Practice Exam-1A Fall 2016
... (a) milk (b) salt, water, and sugar (c) oil and water (d) 14-K gold ring (e) air ...
... (a) milk (b) salt, water, and sugar (c) oil and water (d) 14-K gold ring (e) air ...
Chapter 2 – Atoms and Elements - U of L Class Index
... proton. The problem with this model is that circular motion corresponds to constant acceleration since the particle is constantly changing direction. Acceleration of a charged particle should result in the continuous release of energy as either heat or electromagnetic radiation. As such, the electro ...
... proton. The problem with this model is that circular motion corresponds to constant acceleration since the particle is constantly changing direction. Acceleration of a charged particle should result in the continuous release of energy as either heat or electromagnetic radiation. As such, the electro ...
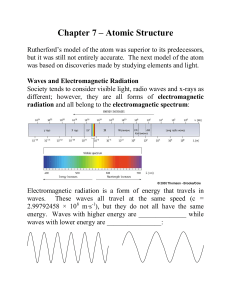
The Nature of Electromagnetic Radiation
... been known that an individual atom is made up of a nucleus (composed of protons and neutrons) and electrons bound to the nucleus, and that the electrons (and hence the atom) have very well defined, discrete amounts of energy. The simplest atom, hydrogen, is composed of a proton (its nucleus) and an ...
... been known that an individual atom is made up of a nucleus (composed of protons and neutrons) and electrons bound to the nucleus, and that the electrons (and hence the atom) have very well defined, discrete amounts of energy. The simplest atom, hydrogen, is composed of a proton (its nucleus) and an ...
PPT
... Light arrives in “packets” of energy (photons). Ephoton = hf Increasing the intensity increases # photons, not the photon energy. Each photon ejects (at most) one electron from the metal. ...
... Light arrives in “packets” of energy (photons). Ephoton = hf Increasing the intensity increases # photons, not the photon energy. Each photon ejects (at most) one electron from the metal. ...
Light, Energy, and More
... Electrons have wavelike motion (because it’s a particle) An electron had restricted orbits Each orbit had a fixed radius from the nucleus Are a wide variety of wavelengths, frequencies, and energies possible? ...
... Electrons have wavelike motion (because it’s a particle) An electron had restricted orbits Each orbit had a fixed radius from the nucleus Are a wide variety of wavelengths, frequencies, and energies possible? ...
C. - Biloxi Public Schools
... • Compare the wave and particle natures of light. • Define a quantum of energy, and explain how it is related to an energy change of matter. • Contrast continuous electromagnetic spectra and atomic emission spectra. radiation: the rays and particles —alpha particles, beta particles, and gamma rays—t ...
... • Compare the wave and particle natures of light. • Define a quantum of energy, and explain how it is related to an energy change of matter. • Contrast continuous electromagnetic spectra and atomic emission spectra. radiation: the rays and particles —alpha particles, beta particles, and gamma rays—t ...
POWERPOINT - Chapter 8
... Divide each molar quantity by the smaller number of moles to get 1 mol for the element with the smaller number of moles. ...
... Divide each molar quantity by the smaller number of moles to get 1 mol for the element with the smaller number of moles. ...
Particle Accelerators - Stony Brook University
... What can we accelerate? All accelerators are based on acceleration of charged particles by electric forces. Electric field E Force F = q E proton of charge q We accelerate elementary particles and nuclei which have small mass (F = ma, so a = F/m) to get beams of high velocity (and energy). Bringing ...
... What can we accelerate? All accelerators are based on acceleration of charged particles by electric forces. Electric field E Force F = q E proton of charge q We accelerate elementary particles and nuclei which have small mass (F = ma, so a = F/m) to get beams of high velocity (and energy). Bringing ...
PHYA1_Rev_3_Q
... one conclusion drawn from these experiments and explain how it supports the particle theory. You may be awarded marks for the quality of written communication in your answer. ...
... one conclusion drawn from these experiments and explain how it supports the particle theory. You may be awarded marks for the quality of written communication in your answer. ...
PPT
... have no basis in physical reality. BUT the model does get some of the numbers right for SIMPLE atoms… ...
... have no basis in physical reality. BUT the model does get some of the numbers right for SIMPLE atoms… ...
Document
... The brilliant red color seen in fireworks displays is due to 4.62 x 1014 s-1 strontium emission. Calculate the wavelength of the light emitted. ...
... The brilliant red color seen in fireworks displays is due to 4.62 x 1014 s-1 strontium emission. Calculate the wavelength of the light emitted. ...
Bremsstrahlung
Bremsstrahlung (German pronunciation: [ˈbʁɛmsˌʃtʁaːlʊŋ], from bremsen ""to brake"" and Strahlung ""radiation"", i.e. ""braking radiation"" or ""deceleration radiation"") is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon, thus satisfying the law of conservation of energy. The term is also used to refer to the process of producing the radiation. Bremsstrahlung has a continuous spectrum, which becomes more intense and whose peak intensity shifts toward higher frequencies as the change of the energy of the accelerated particles increases.Strictly speaking, braking radiation is any radiation due to the acceleration of a charged particle, which includes synchrotron radiation, cyclotron radiation, and the emission of electrons and positrons during beta decay. However, the term is frequently used in the more narrow sense of radiation from electrons (from whatever source) slowing in matter.Bremsstrahlung emitted from plasma is sometimes referred to as free/free radiation. This refers to the fact that the radiation in this case is created by charged particles that are free both before and after the deflection (acceleration) that caused the emission.