thermodynamics
... Mechanics deals with motion of particles under the action of forces, while Thermodynamics concerned with internal Microscopic state of the body. ...
... Mechanics deals with motion of particles under the action of forces, while Thermodynamics concerned with internal Microscopic state of the body. ...
5 Thermochemistry
... 8.0 × 1010 kJ (this is 80 billion kJ!) are released when enough water condenses to produce 0.50 rainfall over an area of one square mile. (b) ...
... 8.0 × 1010 kJ (this is 80 billion kJ!) are released when enough water condenses to produce 0.50 rainfall over an area of one square mile. (b) ...
Chapter 5 Thermochemistry
... of clay is increased when it is moved from the ground to the top of the wall. b) As the ball falls, its potential energy is converted to kinetic energy. c) When it hits the ground, its kinetic energy falls to zero (since it is no longer moving); some of the energy does work on the ball, the rest is ...
... of clay is increased when it is moved from the ground to the top of the wall. b) As the ball falls, its potential energy is converted to kinetic energy. c) When it hits the ground, its kinetic energy falls to zero (since it is no longer moving); some of the energy does work on the ball, the rest is ...
Jeopardy Heat
... What is meant by thermal expansion? The size of an object will change due a temperature Usually, increasing temperature but cause an increase in size. L = LOT where = thermal coefficient in 1/OC ...
... What is meant by thermal expansion? The size of an object will change due a temperature Usually, increasing temperature but cause an increase in size. L = LOT where = thermal coefficient in 1/OC ...
Thermodynamics for Systems Biology
... that no friction is involved. For thermal processes, reversibility requires that all heat move between systems at equal temperatures. For reversible processes, we can construct an additional conservation law as shown below. ...
... that no friction is involved. For thermal processes, reversibility requires that all heat move between systems at equal temperatures. For reversible processes, we can construct an additional conservation law as shown below. ...
chapter 1
... methods of radiotherapy, differential scanning microcalorimetry, methods for separation of molecules (electrophoresis, centrifugation, membrane technology) and so on. The modern physician needs to know not only the structure and functions of the organs of the human body and their pathological distur ...
... methods of radiotherapy, differential scanning microcalorimetry, methods for separation of molecules (electrophoresis, centrifugation, membrane technology) and so on. The modern physician needs to know not only the structure and functions of the organs of the human body and their pathological distur ...
Sec 6.2 Enthalpy - Okemos Public Schools
... temperature goes up from 22⁰ C to 29⁰ C. Calculate ΔH soln by following these steps: a. Use Q = m ∙ c ∙ ΔT to find the about of Joules gained by the water. (Remember to use the mass of the entire solution!) ...
... temperature goes up from 22⁰ C to 29⁰ C. Calculate ΔH soln by following these steps: a. Use Q = m ∙ c ∙ ΔT to find the about of Joules gained by the water. (Remember to use the mass of the entire solution!) ...
Chapter 19
... material that flows from one object to another; it is not. Rather, it is a form of energy. Unit of heat: calorie (cal) 1 cal is the amount of heat necessary to raise the temperature of 1 g of water by 1 Celsius degree. Don’t be fooled—the calories on our food labels are really kilocalories (kcal or ...
... material that flows from one object to another; it is not. Rather, it is a form of energy. Unit of heat: calorie (cal) 1 cal is the amount of heat necessary to raise the temperature of 1 g of water by 1 Celsius degree. Don’t be fooled—the calories on our food labels are really kilocalories (kcal or ...
chem 155 trial questions
... 34. An isolated system is best described by which one of the following statements? a. Neither matter nor heat can pass into or out of the system b. The system has a boundary which allows heat to be transferred but does not allow material to pass into or out of the system c. The system has a diatherm ...
... 34. An isolated system is best described by which one of the following statements? a. Neither matter nor heat can pass into or out of the system b. The system has a boundary which allows heat to be transferred but does not allow material to pass into or out of the system c. The system has a diatherm ...
Chapter 1
... A liquid consists of particles that are in contact with each other, but are able to move past each other in a restricted manner. The particles are in a continuous state of motion, but travel only a fraction of a diameter before bumping into a neighbour. The overriding image is one of movement, but w ...
... A liquid consists of particles that are in contact with each other, but are able to move past each other in a restricted manner. The particles are in a continuous state of motion, but travel only a fraction of a diameter before bumping into a neighbour. The overriding image is one of movement, but w ...
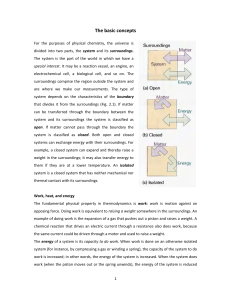
The basic concepts For the purposes of physical chemistry, the
... flows into the system with the lower temperature. If the temperature of either system at thermal equilibrium is raised infinitesimally, then energy flows out of the hotter system. Suppose a gas is confined by a piston and that the external pressure, Pex is set equal to the pressure, P, of the confi ...
... flows into the system with the lower temperature. If the temperature of either system at thermal equilibrium is raised infinitesimally, then energy flows out of the hotter system. Suppose a gas is confined by a piston and that the external pressure, Pex is set equal to the pressure, P, of the confi ...
rocks and minerals quiz
... Matter usually exists in three physical states: solid, liquid, and gas. Solids have a definite shape and a definite volume. Liquids have an indefinite shape and a definite volume. Gases have an indefinite shape and an indefinite volume. STATES OF MATTER Stars in outer space are a notable exception. ...
... Matter usually exists in three physical states: solid, liquid, and gas. Solids have a definite shape and a definite volume. Liquids have an indefinite shape and a definite volume. Gases have an indefinite shape and an indefinite volume. STATES OF MATTER Stars in outer space are a notable exception. ...
Heat transfer

Heat transfer is the exchange of thermal energy between physical systems, depending on the temperature and pressure, by dissipating heat. The fundamental modes of heat transfer are conduction or diffusion, convection and radiation.Heat transfer always occurs from a region of high temperature to another region of lower temperature. Heat transfer changes the internal energy of both systems involved according to the First Law of Thermodynamics. The Second Law of Thermodynamics defines the concept of thermodynamic entropy, by measurable heat transfer.Thermal equilibrium is reached when all involved bodies and the surroundings reach the same temperature. Thermal expansion is the tendency of matter to change in volume in response to a change in temperature.