Chapter 16: Reaction Rates
... Expressing Reaction Rates In the Launch Lab, you discovered that the decomposition of hydrogen peroxide can be a fast reaction, or it can be a slow one. However, fast and slow are inexact terms. Chemists, engineers, chefs, welders, concrete mixers, and others often need to be more specific. For exam ...
... Expressing Reaction Rates In the Launch Lab, you discovered that the decomposition of hydrogen peroxide can be a fast reaction, or it can be a slow one. However, fast and slow are inexact terms. Chemists, engineers, chefs, welders, concrete mixers, and others often need to be more specific. For exam ...
103. Oxalates as Activating Groups for Alcohols in Visible Light Photoredox Catalysis: Formation of Quaternary Centers by Redox-Neutral Fragment Coupling
... are stable and do not readily disproportionate. (11) The cesium oxalates were most conveniently prepared via hydrolysis of the corresponding tert-alkyl methyl oxalates with aqueous CsOH. This route avoids the potentially unstable alkyl hydrogen oxalate moiety; the tert-alkyl methyl oxalate intermedi ...
... are stable and do not readily disproportionate. (11) The cesium oxalates were most conveniently prepared via hydrolysis of the corresponding tert-alkyl methyl oxalates with aqueous CsOH. This route avoids the potentially unstable alkyl hydrogen oxalate moiety; the tert-alkyl methyl oxalate intermedi ...
Document
... 12. Write the IUPAC name of the compound when 1-butene undergoes the process of hydration. 13. Write the IUPAC name of the compound formed by the reaction between propene and hydrogen iodide. Essay 14. Alkanes are compounds of carbon and hydrogen. a Write the molecular formula of an alkane having fi ...
... 12. Write the IUPAC name of the compound when 1-butene undergoes the process of hydration. 13. Write the IUPAC name of the compound formed by the reaction between propene and hydrogen iodide. Essay 14. Alkanes are compounds of carbon and hydrogen. a Write the molecular formula of an alkane having fi ...
CB document - mvhs
... of itself does not release energy. Energy release occurs when new bonds are formed. If more energy is released when new bonds form than was required to break existing bonds, then the difference will result in an overall release of energy. If, on the other hand, more energy is required to break exist ...
... of itself does not release energy. Energy release occurs when new bonds are formed. If more energy is released when new bonds form than was required to break existing bonds, then the difference will result in an overall release of energy. If, on the other hand, more energy is required to break exist ...
PPT - Gmu
... The 2 electrons in filled sp3 orbitals of alkyl groups can overlap with empty p-orbitals of positively charged adjacent Carbon atoms that produces a stabilizing affect on the Carbocation (Hyperconjugation). Electron density shifts toward the positive charge The C-H & C-C orbitals adjacent to the ...
... The 2 electrons in filled sp3 orbitals of alkyl groups can overlap with empty p-orbitals of positively charged adjacent Carbon atoms that produces a stabilizing affect on the Carbocation (Hyperconjugation). Electron density shifts toward the positive charge The C-H & C-C orbitals adjacent to the ...
Aldehydes and Ketones
... Aldehyde are usually more reactive toward nucleophilic substitutions than ketones because of both steric and electronic effects. In aldehydes, the relatively small hydrogen atom is attached to one side of the carbonyl group. While a larger ...
... Aldehyde are usually more reactive toward nucleophilic substitutions than ketones because of both steric and electronic effects. In aldehydes, the relatively small hydrogen atom is attached to one side of the carbonyl group. While a larger ...
Standard C-1: The student will demonstrate an understanding of
... rate determining (slow step) in a reaction mechanism and the rate equation - Understand AND be able to interpret graphical data relating to ...
... rate determining (slow step) in a reaction mechanism and the rate equation - Understand AND be able to interpret graphical data relating to ...
Chapter 24. Amines
... • Primary, secondary, and tertiary amines all have similar reactivity, the initially formed monoalkylated substance undergoes further reaction to yield a mixture of products ...
... • Primary, secondary, and tertiary amines all have similar reactivity, the initially formed monoalkylated substance undergoes further reaction to yield a mixture of products ...
Department of Chemistry Course Description
... (3 Credit Hours) Prerequisite: (03031221 + 0303341) Basic principles of quantum chemistry; simple harmonic motion; the rigid rotor; atomic and molecular structure; basic principles of vibrational, rotational, Raman, and electronic spectra of molecules; chemical bond: molecular orbital theory and LCA ...
... (3 Credit Hours) Prerequisite: (03031221 + 0303341) Basic principles of quantum chemistry; simple harmonic motion; the rigid rotor; atomic and molecular structure; basic principles of vibrational, rotational, Raman, and electronic spectra of molecules; chemical bond: molecular orbital theory and LCA ...
Chapter 24. Amines
... • Primary, secondary, and tertiary amines all have similar reactivity, the initially formed monoalkylated substance undergoes further reaction to yield a mixture of products ...
... • Primary, secondary, and tertiary amines all have similar reactivity, the initially formed monoalkylated substance undergoes further reaction to yield a mixture of products ...
chemical equilibrium
... Adding a catalyst DOES NOT AFFECT THE POSITION OF EQUILIBRIUM. However, it does increase the rate of attainment of equilibrium. This is especially important in reversible, exothermic industrial reactions such as the Haber or Contact Processes where economic factors are paramount. Catalysts provide a ...
... Adding a catalyst DOES NOT AFFECT THE POSITION OF EQUILIBRIUM. However, it does increase the rate of attainment of equilibrium. This is especially important in reversible, exothermic industrial reactions such as the Haber or Contact Processes where economic factors are paramount. Catalysts provide a ...
Chemical Equilibria - Beck-Shop
... to if the system is at equilibrium. Thus, we expect a higher effective collision frequency for the forward reaction than that for the backward reaction. As a result, more products form and the position of equilibrium shifts towards the product side. Q: But as more products form, wouldn’t the rate of ...
... to if the system is at equilibrium. Thus, we expect a higher effective collision frequency for the forward reaction than that for the backward reaction. As a result, more products form and the position of equilibrium shifts towards the product side. Q: But as more products form, wouldn’t the rate of ...
Chemistry 120
... U is a function of the state of the material only, not of the history of the sample or the path taken to prepare the state of the sample. Heat is the transfer of energy between the surroundings and the sample - the symbol for heat is q Work is the result of a force acting over a distance - the symbo ...
... U is a function of the state of the material only, not of the history of the sample or the path taken to prepare the state of the sample. Heat is the transfer of energy between the surroundings and the sample - the symbol for heat is q Work is the result of a force acting over a distance - the symbo ...
Thermodynamics: Entropy and Free Energy
... + oxygen gas. Energy is conserved whether the process runs backward or forward, but there is an allowed direction in which these events always occur. In fact, most chemical and physical changes naturally occur in one direction and can occur in the opposite direction only with assistance. For example ...
... + oxygen gas. Energy is conserved whether the process runs backward or forward, but there is an allowed direction in which these events always occur. In fact, most chemical and physical changes naturally occur in one direction and can occur in the opposite direction only with assistance. For example ...
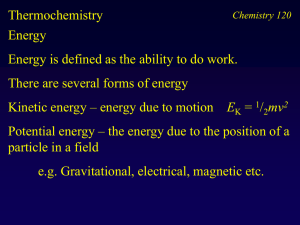
Thermochemistry Energy Energy is defined as the ability to do work
... A sample with a known temperature is placed into a fluid of known heat capacity and known temperature and allowed to come to thermal equilibrium. At thermal equilibrium, Tsample = T fluid and so we know ? T for the sample and for the fluid. We also know C fluid and therefore we know q fluid, the hea ...
... A sample with a known temperature is placed into a fluid of known heat capacity and known temperature and allowed to come to thermal equilibrium. At thermal equilibrium, Tsample = T fluid and so we know ? T for the sample and for the fluid. We also know C fluid and therefore we know q fluid, the hea ...
Kinetics and Equilibrium of the Reversible Formic Acid
... decarbonylation is reversible and that carbon monoxide can be directly converted into formic acid in hot water [5c]. We also made a theoretical analysis on the Gibbs energies of the species (HCOOH, CO, CO2, H2, and H2O) involved in reactions (3) and (4) [5d]. The reversibility and the coupling of th ...
... decarbonylation is reversible and that carbon monoxide can be directly converted into formic acid in hot water [5c]. We also made a theoretical analysis on the Gibbs energies of the species (HCOOH, CO, CO2, H2, and H2O) involved in reactions (3) and (4) [5d]. The reversibility and the coupling of th ...