Name: 1) At 1 atmosphere and 298 K, 1 mole of H O(l) molecules
... Using your knowledge of chemistry and the information in the Vapor Pressure of Four Liquids chemistry reference table, which statement concerning propanone and water at 50DC is true? A) Propanone has a lower vapor pressure and weaker intermolecular forces than water. B) Propanone has a lower vapor p ...
... Using your knowledge of chemistry and the information in the Vapor Pressure of Four Liquids chemistry reference table, which statement concerning propanone and water at 50DC is true? A) Propanone has a lower vapor pressure and weaker intermolecular forces than water. B) Propanone has a lower vapor p ...
Lecture 14a - UCLA Chemistry and Biochemistry
... (NMe2, NEt2): solution method works, but it is very slow; the microwave reaction is much faster The student has to be much more cautious ...
... (NMe2, NEt2): solution method works, but it is very slow; the microwave reaction is much faster The student has to be much more cautious ...
AP Syllabus 95-96 - Bremen High School District 228
... Advanced Placement Chemistry is designed to be the equivalent of the general chemistry course usually taken during the first year of college. Students should attain an understanding of the fundamental principles of chemistry appropriate for this level. They should also achieve competence in using ca ...
... Advanced Placement Chemistry is designed to be the equivalent of the general chemistry course usually taken during the first year of college. Students should attain an understanding of the fundamental principles of chemistry appropriate for this level. They should also achieve competence in using ca ...
4-Physical Chemistry of SW-Equilibrium-ion
... formation) is equal to the rate of the reverse reaction (reactant formation), and the concentrations of all species is constant with time. This is not a static condition - rather it is a dynamic equilibrium - forward and reverse reaction rates balance. The relative concentrations of products [C ]c [ ...
... formation) is equal to the rate of the reverse reaction (reactant formation), and the concentrations of all species is constant with time. This is not a static condition - rather it is a dynamic equilibrium - forward and reverse reaction rates balance. The relative concentrations of products [C ]c [ ...
g) Chemistry 30 - Mr. Jones LHS Science
... c. Draw a reaction coordinate diagram showing if the relative energy of the reactants and products. . Label the ∆H on the diagram. ...
... c. Draw a reaction coordinate diagram showing if the relative energy of the reactants and products. . Label the ∆H on the diagram. ...
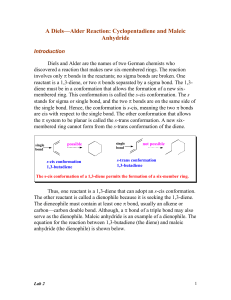
Lab 2 - Academic Computer Center
... The following box shows typical dienes for Diels-Alder reactions. ...
... The following box shows typical dienes for Diels-Alder reactions. ...
Types of Chemical Reactions
... Thousands of known chemical reactions occur in various systems. Memorizing the equations for so many chemical reactions would be difficult. It is more useful and realistic to classify reactions according to various similarities and regularities. ...
... Thousands of known chemical reactions occur in various systems. Memorizing the equations for so many chemical reactions would be difficult. It is more useful and realistic to classify reactions according to various similarities and regularities. ...
CHEMISTRY 112 LECTURE
... Hydrocarbons Hydrocarbons are the simplest of organic compounds, they contain only hydrogen and carbon. ...
... Hydrocarbons Hydrocarbons are the simplest of organic compounds, they contain only hydrogen and carbon. ...
Secondary alcohols
... Rearrangement of an initial secondary carbocation to the more stable tertiary carbocation by a hydride shift results in a rearranged product. ...
... Rearrangement of an initial secondary carbocation to the more stable tertiary carbocation by a hydride shift results in a rearranged product. ...
Chemistry
... Rate of reaction,. Definition of order and molecularity. Derivation of rate constants for first, second, third and zero order reactions and examples. Derivation for time half change. Methods to determine the order of reactions.. Effect of temperature on rate of reaction, Arrhenius equation, concept ...
... Rate of reaction,. Definition of order and molecularity. Derivation of rate constants for first, second, third and zero order reactions and examples. Derivation for time half change. Methods to determine the order of reactions.. Effect of temperature on rate of reaction, Arrhenius equation, concept ...
Naming Ethers Naming Ethers
... • Skunks use thiols as a defense mechanism: (E)-2butene-1-thiol, 3-methyl-1-butanethiol, and 2quinolinemethanethiol, and acetate thioesters of these. • Methanethiol is added to natural gas (methane) so that gas leaks can be detected. • The hydrosulfide ion (HS–) is a strong nucleophile and a weak ...
... • Skunks use thiols as a defense mechanism: (E)-2butene-1-thiol, 3-methyl-1-butanethiol, and 2quinolinemethanethiol, and acetate thioesters of these. • Methanethiol is added to natural gas (methane) so that gas leaks can be detected. • The hydrosulfide ion (HS–) is a strong nucleophile and a weak ...
Investigating Esters
... Esters can also be prepared by reacting an acyl chloride or an anhydride with the carboxylic acid. For example if you were preparing an ester using ethanol as the alcohol this could be replaced by ethanoyl chloride or ethanoic anhydride. The methods of preparation using these reactants are different ...
... Esters can also be prepared by reacting an acyl chloride or an anhydride with the carboxylic acid. For example if you were preparing an ester using ethanol as the alcohol this could be replaced by ethanoyl chloride or ethanoic anhydride. The methods of preparation using these reactants are different ...