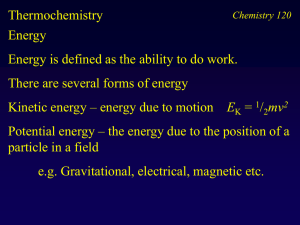
Chm 118
... minimum energy of x; the second, call it B, requires 10x. As temperature is increased (what does he mean by that?) which internal system, A or B, becomes activated first? If a given amount of heat is added to the table (what does he mean by that?) does it go entirely to translational energy? If not, ...
... minimum energy of x; the second, call it B, requires 10x. As temperature is increased (what does he mean by that?) which internal system, A or B, becomes activated first? If a given amount of heat is added to the table (what does he mean by that?) does it go entirely to translational energy? If not, ...
Thermodynamics - cloudfront.net
... Module 2 - Heat Transfer This module describes conduction, convection, and radiation heat transfer. The module also explains how specific parameters can affect the rate of heat transfer. Volume 3 of 3 Module 3 - Fluid Flow This module describes the relationship between the different types of energy ...
... Module 2 - Heat Transfer This module describes conduction, convection, and radiation heat transfer. The module also explains how specific parameters can affect the rate of heat transfer. Volume 3 of 3 Module 3 - Fluid Flow This module describes the relationship between the different types of energy ...
Thermodynamic Processes
... shelf. Due to the friction between the piston and cylinder that occurs in any real process, the piston will not move initially. Moving additional weights from the platform to the shelf will cause the piston to break free and overcome the restraining friction forces. The piston will then accelerate u ...
... shelf. Due to the friction between the piston and cylinder that occurs in any real process, the piston will not move initially. Moving additional weights from the platform to the shelf will cause the piston to break free and overcome the restraining friction forces. The piston will then accelerate u ...
Magnetic properties of Ce compounds studied by specific heat
... the main reasons, why is the specific heat study becoming a standard research tool. In principle, any temperature-dependent phenomenon can contribute to the specific heat of a system since it affects the energy level of particles or modes that determine the mean energy. These levels may arise from t ...
... the main reasons, why is the specific heat study becoming a standard research tool. In principle, any temperature-dependent phenomenon can contribute to the specific heat of a system since it affects the energy level of particles or modes that determine the mean energy. These levels may arise from t ...
Thermodynamic Cycles Knowledge Check
... We have not yet discussed processes performed by gases as we have focused on the steam cycle, yet many applications of the use of gases are occurring all the time during plant operation. The compression of a gas results in different final states than the compression of a saturated vapor such as stea ...
... We have not yet discussed processes performed by gases as we have focused on the steam cycle, yet many applications of the use of gases are occurring all the time during plant operation. The compression of a gas results in different final states than the compression of a saturated vapor such as stea ...
Thermodynamic Cycles Knowledge Check
... We have not yet discussed processes performed by gases as we have focused on the steam cycle, yet many applications of the use of gases are occurring all the time during plant operation. The compression of a gas results in different final states than the compression of a saturated vapor such as stea ...
... We have not yet discussed processes performed by gases as we have focused on the steam cycle, yet many applications of the use of gases are occurring all the time during plant operation. The compression of a gas results in different final states than the compression of a saturated vapor such as stea ...
Thermodynamic Cycles Knowledge Check
... We have not yet discussed processes performed by gases as we have focused on the steam cycle, yet many applications of the use of gases are occurring all the time during plant operation. The compression of a gas results in different final states than the compression of a saturated vapor such as stea ...
... We have not yet discussed processes performed by gases as we have focused on the steam cycle, yet many applications of the use of gases are occurring all the time during plant operation. The compression of a gas results in different final states than the compression of a saturated vapor such as stea ...
The Second Law of Thermodynamics
... its own; that is, a ball sitting on the floor spontaneously rises to a certain height in the air by absorbing heat from the floor. Such a process will not violate the first law. If the mass of the ball is m, and the height above the floor to which it rises is h, we have energy extracted from the floor ¼ ...
... its own; that is, a ball sitting on the floor spontaneously rises to a certain height in the air by absorbing heat from the floor. Such a process will not violate the first law. If the mass of the ball is m, and the height above the floor to which it rises is h, we have energy extracted from the floor ¼ ...
GAS PROCESSES - Elements of Heat Engines
... Processes stated above, except throttling, are considered to be reversible* non flow processes, and throttling process is an irreversible flow process. Many of the above thermodynamic processes can be represented graphically by plotting simultaneous values of pressure and volume. Curves, thus, obtai ...
... Processes stated above, except throttling, are considered to be reversible* non flow processes, and throttling process is an irreversible flow process. Many of the above thermodynamic processes can be represented graphically by plotting simultaneous values of pressure and volume. Curves, thus, obtai ...
Chp-1. Pure substances
... Extensive property of a pure or single substance will depend upon the number of moles (n) of the substance present & also on any two of the three variables- P,V & T (called independent variables). If ‘n’ is kept constant, the extensive property of a system will depend only on the two independent var ...
... Extensive property of a pure or single substance will depend upon the number of moles (n) of the substance present & also on any two of the three variables- P,V & T (called independent variables). If ‘n’ is kept constant, the extensive property of a system will depend only on the two independent var ...
Modern Industrial Assessment1
... 2. A high flue gas temperature often reflects the existence of deposits and fouling on the fire and/or water side(s) of the boiler. The resulting loss in boiler efficiency can be closely estimated on the basis that a 1-% efficiency loss occurs with every 400F increase in stack temperature. It is sug ...
... 2. A high flue gas temperature often reflects the existence of deposits and fouling on the fire and/or water side(s) of the boiler. The resulting loss in boiler efficiency can be closely estimated on the basis that a 1-% efficiency loss occurs with every 400F increase in stack temperature. It is sug ...
Statistical and Low Temperature Physics (PHYS393)
... Through the sound velocity v in the formula for ωD above, the Debye temperature is also directly related to the bond strength and mass of the atoms. For example, for diamond it is 2000 K, and for lead it is 95 K. Liquid helium is unusual in that it is a liquid below its Debye temperature. One possib ...
... Through the sound velocity v in the formula for ωD above, the Debye temperature is also directly related to the bond strength and mass of the atoms. For example, for diamond it is 2000 K, and for lead it is 95 K. Liquid helium is unusual in that it is a liquid below its Debye temperature. One possib ...
Heat

In physics, heat is energy in a process of transfer between a system and its surroundings, other than as work or with the transfer of matter. When there is a suitable physical pathway, heat flows from a hotter body to a colder one. The pathway can be direct, as in conduction and radiation, or indirect, as in convective circulation.Because it refers to a process of transfer between two systems, the system of interest, and its surroundings considered as a system, heat is not a state or property of a single system. If heat transfer is slow and continuous, so that the temperature of the system of interest remains well defined, it can sometimes be described by a process function.Kinetic theory explains heat as a macroscopic manifestation of the motions and interactions of microscopic constituents such as molecules and photons.In calorimetry, sensible heat is defined with respect to a specific chosen state variable of the system, such as pressure or volume. Sensible heat transferred into or out of the system under study causes change of temperature while leaving the chosen state variable unchanged. Heat transfer that occurs with the system at constant temperature and that does change that particular state variable is called latent heat with respect to that variable. For infinitesimal changes, the total incremental heat transfer is then the sum of the latent and sensible heat increments. This is a basic paradigm for thermodynamics, and was important in the historical development of the subject.The quantity of energy transferred as heat is a scalar expressed in an energy unit such as the joule (J) (SI), with a sign that is customarily positive when a transfer adds to the energy of a system. It can be measured by calorimetry, or determined by calculations based on other quantities, relying on the first law of thermodynamics.