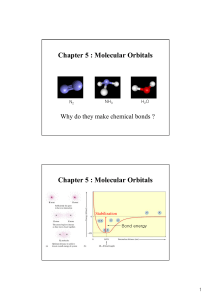
Stabilization of Rock Salt ZnO Nanocrystals by Low
... the surrounding cations. The charges on both anions and cations in the RS phase are consistently higher than the charges on the atoms in the ZB and WZ phases, which is most likely due to the fact that, in the RS phase, the number of interactions with neighboring atoms is larger as all atoms are in s ...
... the surrounding cations. The charges on both anions and cations in the RS phase are consistently higher than the charges on the atoms in the ZB and WZ phases, which is most likely due to the fact that, in the RS phase, the number of interactions with neighboring atoms is larger as all atoms are in s ...
Neutron Scattering—A Non-destructive Microscope for Seeing
... How can we determine the relative positions and motions of atoms in a bulk sample of solid or liquid? Somehow we need to be able to see inside the sample with a suitable magnifying glass. It turns out that neutrons provide us with this capability. They have no charge, and their electric dipole momen ...
... How can we determine the relative positions and motions of atoms in a bulk sample of solid or liquid? Somehow we need to be able to see inside the sample with a suitable magnifying glass. It turns out that neutrons provide us with this capability. They have no charge, and their electric dipole momen ...
BASIC CALORIMETRY SET
... * If a steam generator is not available, a distillation flask and Bunsen burner is adequate. A second flask can be used as a water trap. Introduction When a substance changes phase, the arrangement of its molecules changes. If the new arrangement has a higher internal energy, the substance must abso ...
... * If a steam generator is not available, a distillation flask and Bunsen burner is adequate. A second flask can be used as a water trap. Introduction When a substance changes phase, the arrangement of its molecules changes. If the new arrangement has a higher internal energy, the substance must abso ...
Light-absorption effect on Bragg interference in multilayer semiconductor heterostructures
... noting from Fig. 4 that the Bragg mirror containing 10 periods of a superlattice has roughly the same peak value of the reflection coefficient as an infinite Bragg mirror. The saturation value of the reflection coefficient R ` as a function of k A is shown in Fig. 5. This dependence is nonmonotonic. ...
... noting from Fig. 4 that the Bragg mirror containing 10 periods of a superlattice has roughly the same peak value of the reflection coefficient as an infinite Bragg mirror. The saturation value of the reflection coefficient R ` as a function of k A is shown in Fig. 5. This dependence is nonmonotonic. ...
Absorption Techniques in X
... Arabic numerals 1, 2, 3, . . . have more recently been used in the subscript rather than Roman numerals I, II, III, . . . The electron configuration of one electron deficiency from the 2p orbital is expressed as 1s2 2s2 2p5 3s2 3p6 for an Ar atom. This state has two energy levels corresponding to j ...
... Arabic numerals 1, 2, 3, . . . have more recently been used in the subscript rather than Roman numerals I, II, III, . . . The electron configuration of one electron deficiency from the 2p orbital is expressed as 1s2 2s2 2p5 3s2 3p6 for an Ar atom. This state has two energy levels corresponding to j ...
Chapter 6
... top of the lower stops. Now 100 kJ of heat is transferred to the gas in the cylinder from a source at 100°C, causing it to expand and to raise the loaded piston until the piston reaches the upper stops, as shown in the figure. At this point, the load is removed, and the gas temperature is observed t ...
... top of the lower stops. Now 100 kJ of heat is transferred to the gas in the cylinder from a source at 100°C, causing it to expand and to raise the loaded piston until the piston reaches the upper stops, as shown in the figure. At this point, the load is removed, and the gas temperature is observed t ...
Thermodynamics By S K Mondal
... 1. Zeroth law of thermodynamics is related to temperature 2. Entropy is related to first law of thermodynamics 3. Internal energy of an ideal gas is a function of temperature and pressure 4. Van der Waals' equation is related to an ideal gas Which of the above statements is/are correct? (a) 1 only ( ...
... 1. Zeroth law of thermodynamics is related to temperature 2. Entropy is related to first law of thermodynamics 3. Internal energy of an ideal gas is a function of temperature and pressure 4. Van der Waals' equation is related to an ideal gas Which of the above statements is/are correct? (a) 1 only ( ...
Lecture 4
... • A laser machine consists of the laser, some mirrors or a fiber for beam guidance, focusing optics and a positioning system. The laser beam is focused onto the work-piece and can be moved relatively to it. The laser machining process is controlled by switching the laser on and off, changing the las ...
... • A laser machine consists of the laser, some mirrors or a fiber for beam guidance, focusing optics and a positioning system. The laser beam is focused onto the work-piece and can be moved relatively to it. The laser machining process is controlled by switching the laser on and off, changing the las ...
Calculus-Based Physics I
... to be read/said: ‘x’ equals minus ‘b’, plus-or-minus the square root of ‘b’ squared minus four ‘a’ ‘c’, all over two ‘a’. So, how do you know when you have to use the quadratic formula? There is a good chance that you need it when the square of the variable for which you are solving, appears in the ...
... to be read/said: ‘x’ equals minus ‘b’, plus-or-minus the square root of ‘b’ squared minus four ‘a’ ‘c’, all over two ‘a’. So, how do you know when you have to use the quadratic formula? There is a good chance that you need it when the square of the variable for which you are solving, appears in the ...
Full Text
... formation for sulfuric acid included SO2 formation among side reactions, an additional H = -2.956 kcal/gram SO2 (10) was included as part of the experimental corrections. The problem lies in the Parr assumption that all of the sulfur in the sample is transformed into sulfuric acid. Given a large ex ...
... formation for sulfuric acid included SO2 formation among side reactions, an additional H = -2.956 kcal/gram SO2 (10) was included as part of the experimental corrections. The problem lies in the Parr assumption that all of the sulfur in the sample is transformed into sulfuric acid. Given a large ex ...
Engineering Model to Calculate Mass Flow Rate of a Two
... 2004, SpaceShipOne won the Ansari X Prize after sending the first human to space via a privately funded spacecraft [3]. Going forward the Scaled Composites team has partnered with Virgin GALACTIC becoming the world’s first space tourism company with initial flights planned for 2012. Like SpaceShipOn ...
... 2004, SpaceShipOne won the Ansari X Prize after sending the first human to space via a privately funded spacecraft [3]. Going forward the Scaled Composites team has partnered with Virgin GALACTIC becoming the world’s first space tourism company with initial flights planned for 2012. Like SpaceShipOn ...
A Pool Boiling Map: Water on a Horizontal Surface at
... For analysis, semi-infinite geometries as illustrated in Figure II-1 are initially considered. ...
... For analysis, semi-infinite geometries as illustrated in Figure II-1 are initially considered. ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.