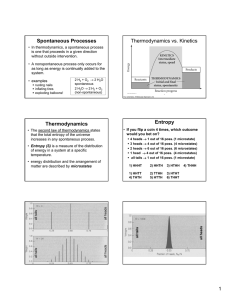
Spontaneous Processes Thermodynamics vs. Kinetics
... microstates (HTTH, HHTT, TTHH, THTH, etc.) ...
... microstates (HTTH, HHTT, TTHH, THTH, etc.) ...
Chemistry Curriculum Overview
... o The energy change within the system is accounted for by the change in the bond energies of the reactants and products. (Note: This does not include calculating the total bond energy changes.) o Breaking bonds requires an input of energy from the system or surroundings, and forming bonds releases e ...
... o The energy change within the system is accounted for by the change in the bond energies of the reactants and products. (Note: This does not include calculating the total bond energy changes.) o Breaking bonds requires an input of energy from the system or surroundings, and forming bonds releases e ...
Honors Chemistry 2 Chapter 10 Test Review
... 39) Using the data tables in your book for standard free energies of formation, determine the free energy change in the following reaction and report whether the reaction will proceed spontaneously: NO(g) + H2O(l) NO2(g) + H2(g) ...
... 39) Using the data tables in your book for standard free energies of formation, determine the free energy change in the following reaction and report whether the reaction will proceed spontaneously: NO(g) + H2O(l) NO2(g) + H2(g) ...
lec12 - UConn Physics
... is turned into heat using friction in the brakes. The total energy of the “car-breaks-road-atmosphere” system is the same. The energy of the car “alone” is not conserved... » It is reduced by the braking. ...
... is turned into heat using friction in the brakes. The total energy of the “car-breaks-road-atmosphere” system is the same. The energy of the car “alone” is not conserved... » It is reduced by the braking. ...
Apply knowledge of fundamental engineering science
... knowledge of statics in engineering science, apply knowledge of energy and momentum, perform calculations related to work, power and efficiency, apply knowledge related to mechanical drives and lifting machines, apply knowledge of friction, apply fundamental knowledge of heat, demonstrate fundamenta ...
... knowledge of statics in engineering science, apply knowledge of energy and momentum, perform calculations related to work, power and efficiency, apply knowledge related to mechanical drives and lifting machines, apply knowledge of friction, apply fundamental knowledge of heat, demonstrate fundamenta ...
AP Chemistry
... higher energy state than reactants (making the product bonds weaker than the reactant bonds) and energy of the system increases (+H), which is described as endothermic because the surroundings typically lose heat energy and cool down. Alternatively, when energy required to break bonds is less than ...
... higher energy state than reactants (making the product bonds weaker than the reactant bonds) and energy of the system increases (+H), which is described as endothermic because the surroundings typically lose heat energy and cool down. Alternatively, when energy required to break bonds is less than ...
Physics 225 Relativity and Math Applications Unit 5 E = mc
... Our study of relativistic dynamics also starts with light. As you will see in Physics 212, Maxwell’s equations describe what light is: it is a particular configuration of electric and magnetic fields propagating through space at speed c. Seriously, light is just force-fields! It hardly seems real at ...
... Our study of relativistic dynamics also starts with light. As you will see in Physics 212, Maxwell’s equations describe what light is: it is a particular configuration of electric and magnetic fields propagating through space at speed c. Seriously, light is just force-fields! It hardly seems real at ...
Introduction to Thermochemistry and Specific Heat
... • The amount of heat given off for one mole of a substance during a phase transition while cooling is its molar enthalpy of transition (DHcond, DHsol, DHdep). What is the sign for all three? -DH • The shape of a heating curve depends upon the heating rate, specific heat capacities of the phases invo ...
... • The amount of heat given off for one mole of a substance during a phase transition while cooling is its molar enthalpy of transition (DHcond, DHsol, DHdep). What is the sign for all three? -DH • The shape of a heating curve depends upon the heating rate, specific heat capacities of the phases invo ...
Continuous System Modeling - ETH
... • The resistor converts free energy irreversibly into entropy. • This fact is represented in the bond graph by a resistive source, the RS-element. • The causality of the thermal side is always such that the resistor is seen there as a source of entropy, never as a source of temperature. • Sources of ...
... • The resistor converts free energy irreversibly into entropy. • This fact is represented in the bond graph by a resistive source, the RS-element. • The causality of the thermal side is always such that the resistor is seen there as a source of entropy, never as a source of temperature. • Sources of ...
Colloidal Crystal: emergence of long range order from colloidal fluid
... not observed, rather a glassy state formed due to the very slow dynamics of the system and frustrated rearrangement of particles to energy minimized states. The phase diagram shows that below a critical effective volume fraction φc = 0.494 [6], (the number from extrapolation of experiment data)no cr ...
... not observed, rather a glassy state formed due to the very slow dynamics of the system and frustrated rearrangement of particles to energy minimized states. The phase diagram shows that below a critical effective volume fraction φc = 0.494 [6], (the number from extrapolation of experiment data)no cr ...
Science of Energy I by Metin C¸ akanyıldırım 1 Forms of Energy 2
... 1 Forms of Energy The laws of science imply that energy and mass are conserved, i.e., a closed system’s energy or mass cannot be increased without an energy or a mass transfer from outside the system. The energy in a closed system, without getting lost, changes from one form to another. Major forms ...
... 1 Forms of Energy The laws of science imply that energy and mass are conserved, i.e., a closed system’s energy or mass cannot be increased without an energy or a mass transfer from outside the system. The energy in a closed system, without getting lost, changes from one form to another. Major forms ...
1a) Charged particles in matter :-
... ii) The electrons revolve around the nucleus in special orbits called discrete orbits. iii) These orbits are called shells or energy levels and are represented by the letters K, L, M, N etc. or numbered as 1, 2, 3, 4, etc. iv) While revolving in the discrete orbits the electrons do not radiate energ ...
... ii) The electrons revolve around the nucleus in special orbits called discrete orbits. iii) These orbits are called shells or energy levels and are represented by the letters K, L, M, N etc. or numbered as 1, 2, 3, 4, etc. iv) While revolving in the discrete orbits the electrons do not radiate energ ...
CHAPTER 13 LEARNING OBJECTIVES - crypt
... Q5. Combine the expression from the previous 2 questions to show that the kinetic energy of the gas is given by KE = 3/2 PV. Q6. The KE of a substance is proportional to its temperature T. Use this to show that PV α T as in the experimental ideal gas law. Q7. Show that, for molar quantities, KE = 3/ ...
... Q5. Combine the expression from the previous 2 questions to show that the kinetic energy of the gas is given by KE = 3/2 PV. Q6. The KE of a substance is proportional to its temperature T. Use this to show that PV α T as in the experimental ideal gas law. Q7. Show that, for molar quantities, KE = 3/ ...
NTD_Final_Ch3-1_3-2 DOWNLOAD
... the heating coil upward, a slow displacement of the molten zone takes place followed by solidification. Once the whole polycrystalline ingot has been passed through by the molten zone, a single crystal ingot is obtained. By repeating this process one can obtain an extremely pure crystal. The ratio o ...
... the heating coil upward, a slow displacement of the molten zone takes place followed by solidification. Once the whole polycrystalline ingot has been passed through by the molten zone, a single crystal ingot is obtained. By repeating this process one can obtain an extremely pure crystal. The ratio o ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.