experimental evaluation of heat exchange between water surface
... and water depths of 5 m the half-life-period amounts to 8 days. Normally constant meteorological conditions can not be expected throughout such long periods. Furthermore an applicable computation of heat exchange coefficients is only possible, if wellknown data about evaporation and condensation are ...
... and water depths of 5 m the half-life-period amounts to 8 days. Normally constant meteorological conditions can not be expected throughout such long periods. Furthermore an applicable computation of heat exchange coefficients is only possible, if wellknown data about evaporation and condensation are ...
Slides
... Temperature reflects the velocity distribution of gas particles The velocity of a single particle constantly changes due to collisions The total number of particles with a given velocity is constant The distribution of molecular speeds in an ideal gas is given by the Maxwell-Boltzmann distribution ...
... Temperature reflects the velocity distribution of gas particles The velocity of a single particle constantly changes due to collisions The total number of particles with a given velocity is constant The distribution of molecular speeds in an ideal gas is given by the Maxwell-Boltzmann distribution ...
Discussion paper on calorific values
... the legal text. This calls for a continued use and update of net values alongside the gross values if the net values are the basis for the calculations. Any legal consequences may need to be discussed further. Since conversion between the different values is done today with a simple factor, a change ...
... the legal text. This calls for a continued use and update of net values alongside the gross values if the net values are the basis for the calculations. Any legal consequences may need to be discussed further. Since conversion between the different values is done today with a simple factor, a change ...
WRL1738.tmp - Symposium on Chemical Physics
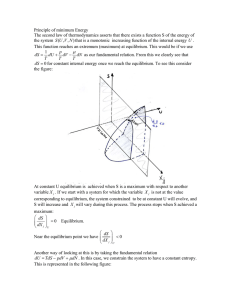
... many systems the process of preparation does matter (e.g. systems showing hysteresis, or glassy materials, see HC), and this means that such systems are not in thermodynamic equilibrium. It is also important that equilibrium thermodynamics only refers to the properties of equilibrium states, and do ...
... many systems the process of preparation does matter (e.g. systems showing hysteresis, or glassy materials, see HC), and this means that such systems are not in thermodynamic equilibrium. It is also important that equilibrium thermodynamics only refers to the properties of equilibrium states, and do ...
1 Thermodynamics All biochemical and cellular processes obey the
... important for analyzing any chemical or biological system. However, the ∆G only determines the direction in which a process will occur, and does not include the time required for a process to occur. ∆G is a measure of spontaneity, but ∆G is totally unrelated to rate. A reaction may have a very large ...
... important for analyzing any chemical or biological system. However, the ∆G only determines the direction in which a process will occur, and does not include the time required for a process to occur. ∆G is a measure of spontaneity, but ∆G is totally unrelated to rate. A reaction may have a very large ...
Class notes
... substituting for V = nRT/pi and integrating from a partial pressure of a compound defined as pi0 to pi ig = RT ln pi/pi0 ...
... substituting for V = nRT/pi and integrating from a partial pressure of a compound defined as pi0 to pi ig = RT ln pi/pi0 ...
Chapter 6 Free Electron Fermi Gas
... temperature T, has an average translational kinetic energy of (3/2)kBT, where kB is the Boltzmann constant. As a consequence, since kinetic energy is equal to 1/2(mass)(velocity)2, the heavier atoms of xenon have a lower average speed than do the lighter atoms of helium at the same temperature. • In ...
... temperature T, has an average translational kinetic energy of (3/2)kBT, where kB is the Boltzmann constant. As a consequence, since kinetic energy is equal to 1/2(mass)(velocity)2, the heavier atoms of xenon have a lower average speed than do the lighter atoms of helium at the same temperature. • In ...
Chemistry II
... 58.) By “spontaneous” we don’t mean that the reaction will form products without intervention. Most often some amount of energy must be imparted to get the process started. 59.)The enthalpy change of a reaction does give an indication as to whether the reaction is likely to be ___________________. ...
... 58.) By “spontaneous” we don’t mean that the reaction will form products without intervention. Most often some amount of energy must be imparted to get the process started. 59.)The enthalpy change of a reaction does give an indication as to whether the reaction is likely to be ___________________. ...