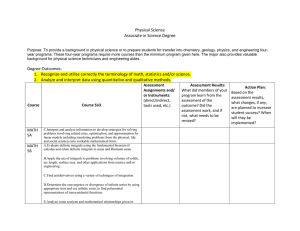
Table of Content
... signifies that the liquid state is far less compressible, compared to the gas state (i.e., points over A-B). Essentially points between X-Y (including Y itself) represent compressed liquid states. An important point to re-emphasize is that on the two-phase line B-X, the pressure of the system remain ...
... signifies that the liquid state is far less compressible, compared to the gas state (i.e., points over A-B). Essentially points between X-Y (including Y itself) represent compressed liquid states. An important point to re-emphasize is that on the two-phase line B-X, the pressure of the system remain ...
Contents - MyCourses
... 2.2 Internal energy U and Maxwell relations . . . . 2.3 Enthalpy H . . . . . . . . . . . . . . . . . . . . . . 2.4 Free energy F . . . . . . . . . . . . . . . . . . . . ...
... 2.2 Internal energy U and Maxwell relations . . . . 2.3 Enthalpy H . . . . . . . . . . . . . . . . . . . . . . 2.4 Free energy F . . . . . . . . . . . . . . . . . . . . ...
Simulation of the influence of preheating on stress - Purdue e-Pubs
... crack. Meanwhile, the inner physics of the transport of heat and force process is very complex, traditional methods based on experience and experimental test is inefficiency and high scrap rate, the adoption of ...
... crack. Meanwhile, the inner physics of the transport of heat and force process is very complex, traditional methods based on experience and experimental test is inefficiency and high scrap rate, the adoption of ...
Power Disipation in CMOS Circuits
... As can be seen from Eq (26.12), the power dissipated can be reduced by reducing either the clock frequency, , or the load capacitance, , or the rail voltage, , or the switching activity parameter, α. Reducing the clock frequency is the easiest thing to do, but it seriously affects the performance o ...
... As can be seen from Eq (26.12), the power dissipated can be reduced by reducing either the clock frequency, , or the load capacitance, , or the rail voltage, , or the switching activity parameter, α. Reducing the clock frequency is the easiest thing to do, but it seriously affects the performance o ...
Thermochemistry
... more abstract but nevertheless useful way, heat is the energy transferred between a system and its surroundings because of their difference in temperature. A combustion reaction, such as the burning of natural gas illustrated in Figure S.l(b), releases the chemical energy stored in the molecules of ...
... more abstract but nevertheless useful way, heat is the energy transferred between a system and its surroundings because of their difference in temperature. A combustion reaction, such as the burning of natural gas illustrated in Figure S.l(b), releases the chemical energy stored in the molecules of ...
Estimatin of Social Entropy - Research India Publications
... This is a complete state of rest or dissolution, with an absence of available energy for doing work. The phenomenon of entropy was originally observed in thermodynamic systems such as heat baths. An isolated system is one that is closed to inputs of both matter and energy. This means that since no n ...
... This is a complete state of rest or dissolution, with an absence of available energy for doing work. The phenomenon of entropy was originally observed in thermodynamic systems such as heat baths. An isolated system is one that is closed to inputs of both matter and energy. This means that since no n ...
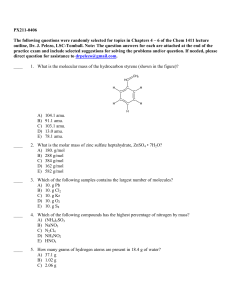
PX211-0406 The following questions were randomly selected for
... 17. When the valve between the 2.00-L bulb, in which the gas pressure is 3.00 atm, and the 3.00-L bulb, in which the gas pressure is 2.50 atm, is opened, what will be the final pressure in the two bulbs? Assume the temperature remains constant. ...
... 17. When the valve between the 2.00-L bulb, in which the gas pressure is 3.00 atm, and the 3.00-L bulb, in which the gas pressure is 2.50 atm, is opened, what will be the final pressure in the two bulbs? Assume the temperature remains constant. ...