GE1 Spinning Charged Ring Model of Electron Yielding Anomalous
... links to a mechanical and physical structure that is the pursuit of physics (the study of the physical nature and properties of the universe). The need for a better electron model is stated by Ivan Sellin who wrote in 1982 that “...a good theory of electron structure still is lacking.... There is st ...
... links to a mechanical and physical structure that is the pursuit of physics (the study of the physical nature and properties of the universe). The need for a better electron model is stated by Ivan Sellin who wrote in 1982 that “...a good theory of electron structure still is lacking.... There is st ...
Poster_IAEA 2000 - Helically Symmetric eXperiment
... evidence of island structures inside the separatrix. The experimental determination of the rotational transform agrees with numerical calculations to within 1%. A simple analytic expression is derived in Boozer coordinates to relate the drift orbits of passing particles to the magnetic field spectru ...
... evidence of island structures inside the separatrix. The experimental determination of the rotational transform agrees with numerical calculations to within 1%. A simple analytic expression is derived in Boozer coordinates to relate the drift orbits of passing particles to the magnetic field spectru ...
Electricity and Magnetism
... There are two different kinds of charged particles, positive (PAH-zuh-tiv) and negative (NEH-guh-tiv). A negative charge comes from electrons. A material that has extra electrons is negatively charged. A positive charge comes from protons. A material that loses electrons is positively charged. There ...
... There are two different kinds of charged particles, positive (PAH-zuh-tiv) and negative (NEH-guh-tiv). A negative charge comes from electrons. A material that has extra electrons is negatively charged. A positive charge comes from protons. A material that loses electrons is positively charged. There ...
Physics 30 - Structured Independent Learning
... B Using the left hand (negative charge), the fingers (B) point to the left and the thumb (e- flow) points down the page – the palm indicates a force which is out of the ...
... B Using the left hand (negative charge), the fingers (B) point to the left and the thumb (e- flow) points down the page – the palm indicates a force which is out of the ...
- Post Graduate Government College
... • The frequency at which a particular proton absorbs is determined by its electronic environment. • The size of the magnetic field generated by the electrons around a proton determines where it absorbs. • Modern NMR spectrometers use a constant magnetic field strength B0, and then a narrow range of ...
... • The frequency at which a particular proton absorbs is determined by its electronic environment. • The size of the magnetic field generated by the electrons around a proton determines where it absorbs. • Modern NMR spectrometers use a constant magnetic field strength B0, and then a narrow range of ...
Activity Lesson Plan
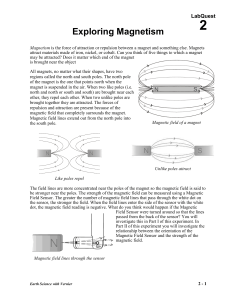
... • explain that like poles repel each other while opposite poles attract each other • describe magnetism as a force with force lines extending from an object into space • recognize that most magnetic objects contain iron (some other less common elements are also magnetic) • demonstrate that iron-cont ...
... • explain that like poles repel each other while opposite poles attract each other • describe magnetism as a force with force lines extending from an object into space • recognize that most magnetic objects contain iron (some other less common elements are also magnetic) • demonstrate that iron-cont ...
Electric Generators and Motors
... connected to a circuit, current will flow in it, and will produce a counter torque. This means the external applied torque must increase to keep the generator turning. ...
... connected to a circuit, current will flow in it, and will produce a counter torque. This means the external applied torque must increase to keep the generator turning. ...
Magnetochemistry

Magnetochemistry is concerned with the magnetic properties of chemical compounds. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaired electrons. Molecular compounds that contain one or more unpaired electrons are paramagnetic. The magnitude of the paramagnetism is expressed as an effective magnetic moment, μeff. For first-row transition metals the magnitude of μeff is, to a first approximation, a simple function of the number of unpaired electrons, the spin-only formula. In general, spin-orbit coupling causes μeff to deviate from the spin-only formula. For the heavier transition metals, lanthanides and actinides, spin-orbit coupling cannot be ignored. Exchange interaction can occur in clusters and infinite lattices, resulting in ferromagnetism, antiferromagnetism or ferrimagnetism depending on the relative orientations of the individual spins.