MAGNETISM
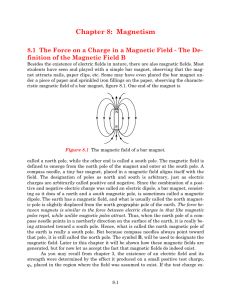
... Analyze the results of the experiment It’s imposible to separate the north and the south pole of a magnet ...
... Analyze the results of the experiment It’s imposible to separate the north and the south pole of a magnet ...
Quantum Mechanics Magnetic field
... the magnetic field around any current-carrying wire. Extending these experiments, Ampère published his own successful model of magnetism in 1825. In it, he showed the equivalence of electrical currents to magnets[7] and proposed that magnetism is due to perpetually flowing loops of current instead o ...
... the magnetic field around any current-carrying wire. Extending these experiments, Ampère published his own successful model of magnetism in 1825. In it, he showed the equivalence of electrical currents to magnets[7] and proposed that magnetism is due to perpetually flowing loops of current instead o ...
Lecture20
... Example: the L of a solenoid of length 10 cm, area 5 cm2, with a total of 100 turns is L = 6.28×10−5 H 0.5 mm diameter wire would achieve 100 turns in a single layer. Going to 10 layers would increase L by a factor of 100. Adding an iron or ferrite core would also increase L by about a factor of 100 ...
... Example: the L of a solenoid of length 10 cm, area 5 cm2, with a total of 100 turns is L = 6.28×10−5 H 0.5 mm diameter wire would achieve 100 turns in a single layer. Going to 10 layers would increase L by a factor of 100. Adding an iron or ferrite core would also increase L by about a factor of 100 ...
Shielding of electromagnetic fields by mono- and multi
... with large conductivity. In this range of frequency epidermical effect appears, in result of which electromagnetic wave is extinguished in depths of half wave’s length [7]. From the point of view of its destination one can distinguish two kinds of electromagnetic field shields [8]: anti-disturbance ...
... with large conductivity. In this range of frequency epidermical effect appears, in result of which electromagnetic wave is extinguished in depths of half wave’s length [7]. From the point of view of its destination one can distinguish two kinds of electromagnetic field shields [8]: anti-disturbance ...
Magnetochemistry

Magnetochemistry is concerned with the magnetic properties of chemical compounds. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaired electrons. Molecular compounds that contain one or more unpaired electrons are paramagnetic. The magnitude of the paramagnetism is expressed as an effective magnetic moment, μeff. For first-row transition metals the magnitude of μeff is, to a first approximation, a simple function of the number of unpaired electrons, the spin-only formula. In general, spin-orbit coupling causes μeff to deviate from the spin-only formula. For the heavier transition metals, lanthanides and actinides, spin-orbit coupling cannot be ignored. Exchange interaction can occur in clusters and infinite lattices, resulting in ferromagnetism, antiferromagnetism or ferrimagnetism depending on the relative orientations of the individual spins.