Page 1 - Madeley High School
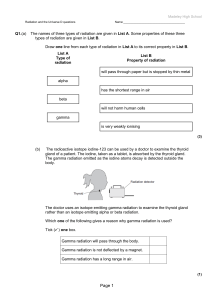
... The doctor uses an isotope emitting gamma radiation to examine the thyroid gland rather than an isotope emitting alpha or beta radiation. Which one of the following gives a reason why gamma radiation is used? Tick ( ) one box. Gamma radiation will pass through the body. Gamma radiation is not deflec ...
... The doctor uses an isotope emitting gamma radiation to examine the thyroid gland rather than an isotope emitting alpha or beta radiation. Which one of the following gives a reason why gamma radiation is used? Tick ( ) one box. Gamma radiation will pass through the body. Gamma radiation is not deflec ...
Nuclear Chemistry - Moorpark College
... combinations: Z even - N even = 163; Z even - N odd 55: Z odd - N even 50 and Z odd -N odd 4. There are no stable nuclides with an atomic number greater than 83. All but two (43Tc and 61Pm) under 83 have at least one stable isotope. A band of stability naturally occurs if you chart the number neutro ...
... combinations: Z even - N even = 163; Z even - N odd 55: Z odd - N even 50 and Z odd -N odd 4. There are no stable nuclides with an atomic number greater than 83. All but two (43Tc and 61Pm) under 83 have at least one stable isotope. A band of stability naturally occurs if you chart the number neutro ...
Lecture Notes - Horizon Medical Institute
... nurse explains that sodium retention causes increased fluid in tissues. The swelling is called ________. A: edema Q: Jane is diagnosed with Cushing syndrome. The physician explains the goal of treatment is to restore concentration of normal levels of the principal steroid hormone produced by the adr ...
... nurse explains that sodium retention causes increased fluid in tissues. The swelling is called ________. A: edema Q: Jane is diagnosed with Cushing syndrome. The physician explains the goal of treatment is to restore concentration of normal levels of the principal steroid hormone produced by the adr ...
SYNTHROID PM
... always take it on an empty stomach (preferred method) or take it with food]. Levothyroxine sodium absorption is increased on an empty stomach. Usual dose: The dose of these medicines will be different for different patients. Follow your doctor's orders or the directions on the label. Treatment is us ...
... always take it on an empty stomach (preferred method) or take it with food]. Levothyroxine sodium absorption is increased on an empty stomach. Usual dose: The dose of these medicines will be different for different patients. Follow your doctor's orders or the directions on the label. Treatment is us ...
DM (STZ/N) - Conferences
... • You need to stimulate the failing heart so we should try positive inotropes! Wrong • You can’t inhibit the failing heart with β-blockersyou will kill them! Wrong again • If you give thyroid hormones to cardiac patients, you will trigger arrhythmias and may kill them! ...
... • You need to stimulate the failing heart so we should try positive inotropes! Wrong • You can’t inhibit the failing heart with β-blockersyou will kill them! Wrong again • If you give thyroid hormones to cardiac patients, you will trigger arrhythmias and may kill them! ...
Graves Disease Booklet
... Radioactive iodine. This treatment, usually a pill taken once by mouth, damages or destroys thyroid cells that produce excess thyroid hormone. This process may take a few weeks or months. The thyroid gland is reduced in size, and the amount of hormone produced is also reduced. People given this trea ...
... Radioactive iodine. This treatment, usually a pill taken once by mouth, damages or destroys thyroid cells that produce excess thyroid hormone. This process may take a few weeks or months. The thyroid gland is reduced in size, and the amount of hormone produced is also reduced. People given this trea ...
Radioactivity Reading Assignment Name: Chemistry Date: Hour
... We don't find pure gamma sources–gamma rays are emitted alongside alpha or beta particles. If we want a source of pure gamma rays, we can get it by using a substance that emits both beta and gamma, and simply keep it in an aluminum container that stops the beta particles. Strictly speaking, gamma em ...
... We don't find pure gamma sources–gamma rays are emitted alongside alpha or beta particles. If we want a source of pure gamma rays, we can get it by using a substance that emits both beta and gamma, and simply keep it in an aluminum container that stops the beta particles. Strictly speaking, gamma em ...
Chapter 32 Applied Nucleonics
... The discovery of nuclear fission in 1938 by Hahn and Strassman suggested the possibility of tapping the energy of the nucleus. Recall that it is a conversion of some of the nuclear binding energy into kinetic energy that characterizes both fission and fusion. The basis of this conversion can be seen ...
... The discovery of nuclear fission in 1938 by Hahn and Strassman suggested the possibility of tapping the energy of the nucleus. Recall that it is a conversion of some of the nuclear binding energy into kinetic energy that characterizes both fission and fusion. The basis of this conversion can be seen ...
Endocrinology II (French)
... • ↑uptake: Graves, toxic multinodular goiter, solitary toxic adenoma • ↓uptake: thyroiditis, factitious hyperthyroidism ...
... • ↑uptake: Graves, toxic multinodular goiter, solitary toxic adenoma • ↓uptake: thyroiditis, factitious hyperthyroidism ...
Absorption and Biological Effects of Ionising Radiation
... neutrons), emitted by the atoms of radioactive elements, such as radium, plutonium and uranium. The process of alpha decay transforms one element into another, decreasing the atomic mass (or nuclear number) by four. Because of their large mass alpha particles have short range of only a few centimetr ...
... neutrons), emitted by the atoms of radioactive elements, such as radium, plutonium and uranium. The process of alpha decay transforms one element into another, decreasing the atomic mass (or nuclear number) by four. Because of their large mass alpha particles have short range of only a few centimetr ...
Levothyroxine - Drs. Foster and Smith
... to change, your veterinarian may need to recheck thyroid hormone levels and adjust dose if indicated. Not for use in animals hypersensitive (allergic) to it. Use with extreme caution in older or debilitated animals, or those with heart disease, high blood pressure, Addison's disease (hypoadrenocorti ...
... to change, your veterinarian may need to recheck thyroid hormone levels and adjust dose if indicated. Not for use in animals hypersensitive (allergic) to it. Use with extreme caution in older or debilitated animals, or those with heart disease, high blood pressure, Addison's disease (hypoadrenocorti ...
Nuclear Chemistry Radioactivity
... – In contrast, nuclides to the right of the band of stability have a neutron-to-proton ratio smaller than that needed for a stable nucleus. – These nuclides tend to decay by positron emission or electron capture because it increases the neutron to proton ratio. – In the very heavy elements, especial ...
... – In contrast, nuclides to the right of the band of stability have a neutron-to-proton ratio smaller than that needed for a stable nucleus. – These nuclides tend to decay by positron emission or electron capture because it increases the neutron to proton ratio. – In the very heavy elements, especial ...
notes ch 39 1st half Atomic Nucleus and Radioactivity
... • Visible light is given off when electrons jump from one orbit to another of lower energy, and gamma rays are emitted when nucleons do a similar sort of thing inside the nucleus. • Gamma rays require lead or other heavy shielding to stop them. ...
... • Visible light is given off when electrons jump from one orbit to another of lower energy, and gamma rays are emitted when nucleons do a similar sort of thing inside the nucleus. • Gamma rays require lead or other heavy shielding to stop them. ...
here - Recovering with T3
... level of adrenocortical activity commensurate with the lowered metabolic state. When thyroid-replacement therapy is administered, the metabolism increases at a greater rate than adrenocortical activity. This can precipitate adrenocortical insufficiency. Therefore, in severe and prolonged hypothyroid ...
... level of adrenocortical activity commensurate with the lowered metabolic state. When thyroid-replacement therapy is administered, the metabolism increases at a greater rate than adrenocortical activity. This can precipitate adrenocortical insufficiency. Therefore, in severe and prolonged hypothyroid ...
The Thyroid Gland: Function and Regulation
... Thyroid Hormones • The thyroid gland is located immediately below the larynx on each side of and anterior to the trachea. • It is one of the largest of the endocrine glands, normally weighing 15 to 20 grams in adults. • The thyroid secretes two major hormones, thyroxine and triiodothyronine, co ...
... Thyroid Hormones • The thyroid gland is located immediately below the larynx on each side of and anterior to the trachea. • It is one of the largest of the endocrine glands, normally weighing 15 to 20 grams in adults. • The thyroid secretes two major hormones, thyroxine and triiodothyronine, co ...
Scholars Bulletin Multicystic ovaries in uncontrolled congenital
... hormone (TSH), follicle stimulating hormone (FSH), luteinizing hormone (LH) have in common alpha chain and it is their chain that confer specifity. Cross-reaction of very high TSH could produce FSH and LH like activity responsible for the luteinized ovarian cysts [9]. In some cases reports the FSH l ...
... hormone (TSH), follicle stimulating hormone (FSH), luteinizing hormone (LH) have in common alpha chain and it is their chain that confer specifity. Cross-reaction of very high TSH could produce FSH and LH like activity responsible for the luteinized ovarian cysts [9]. In some cases reports the FSH l ...
File - Coach Frei Science
... Acromegaly = the facial bones (lower jaw and ridge of brow), hands and feet enlarged ...
... Acromegaly = the facial bones (lower jaw and ridge of brow), hands and feet enlarged ...
Radioactive Decay
... Example: Assuming a half-life of _________, how many years will be needed for the decay of ________ of a given amount of radium-226? Amount remaining = 1/16 = 0.0625 = (½)4 = ____________ Years needed for decay of 15/16 = (1599 years) (__) = ___________ ...
... Example: Assuming a half-life of _________, how many years will be needed for the decay of ________ of a given amount of radium-226? Amount remaining = 1/16 = 0.0625 = (½)4 = ____________ Years needed for decay of 15/16 = (1599 years) (__) = ___________ ...
ST120 Endocrine System
... Overgrowth of the thyroid gland Smooth appearance May or may not cause overproduction of hormones Due to lack of iodine in the diet ...
... Overgrowth of the thyroid gland Smooth appearance May or may not cause overproduction of hormones Due to lack of iodine in the diet ...
Sickle Cell Disease
... Edema around eyes, face and hands Constipation Sleepiness Mental decline-not permanent cognitive impairment ...
... Edema around eyes, face and hands Constipation Sleepiness Mental decline-not permanent cognitive impairment ...
Radioactivity Revision Questions Decay – Nucleus
... 9. How can a Nucleus Be Unstable? A stable atom will have a stable number of neutrons. For small atoms (containing less than 20 protons) a stable atom will have approximately the same number of protons and neutrons. An atom which is very different from this will be unstable. 10. How can an Unstable ...
... 9. How can a Nucleus Be Unstable? A stable atom will have a stable number of neutrons. For small atoms (containing less than 20 protons) a stable atom will have approximately the same number of protons and neutrons. An atom which is very different from this will be unstable. 10. How can an Unstable ...
Nuclear Notation
... from nuclei as a result of nuclear instability. y Because the nucleus experiences the intense conflict between the two strongest forces in nature, it should not be surprising that there are many nuclear isotopes which are unstable and emit some kind of radiation. y The most common types ...
... from nuclei as a result of nuclear instability. y Because the nucleus experiences the intense conflict between the two strongest forces in nature, it should not be surprising that there are many nuclear isotopes which are unstable and emit some kind of radiation. y The most common types ...
Understanding your thyroid function results
... If your blood tests are too high or too low, you will need to talk to your health provider about what treatment you need. If you have an underactive thyroid, this is easily treated with thyroxine tablets that you take every day. If you have an overactive thyroid, there are several ways of treating t ...
... If your blood tests are too high or too low, you will need to talk to your health provider about what treatment you need. If you have an underactive thyroid, this is easily treated with thyroxine tablets that you take every day. If you have an overactive thyroid, there are several ways of treating t ...
Iodine-131
Iodine-131 (131I), also loosely and nonspecifically called radioiodine, is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nuclear energy, medical diagnostic and treatment procedures, and natural gas production. It also plays a major role as a radioactive isotope present in nuclear fission products, and was a significant contributor to the health hazards from open-air atomic bomb testing in the 1950s, and from the Chernobyl disaster, as well as being a large fraction of the contamination hazard in the first weeks in the Fukushima nuclear crisis. This is because I-131 is a major uranium, plutonium fission product, comprising nearly 3% of the total products of fission (by weight). See fission product yield for a comparison with other radioactive fission products. I-131 is also a major fission product of uranium-233, produced from thorium.Due to its mode of beta decay, iodine-131 is notable for causing mutation and death in cells that it penetrates, and other cells up to several millimeters away. For this reason, high doses of the isotope are sometimes less dangerous than low doses, since they tend to kill thyroid tissues that would otherwise become cancerous as a result of the radiation. For example, children treated with moderate dose of I-131 for thyroid adenomas had a detectable increase in thyroid cancer, but children treated with a much higher dose did not. Likewise, most studies of very-high-dose I-131 for treatment of Graves disease have failed to find any increase in thyroid cancer, even though there is linear increase in thyroid cancer risk with I-131 absorption at moderate doses. Thus, iodine-131 is increasingly less employed in small doses in medical use (especially in children), but increasingly is used only in large and maximal treatment doses, as a way of killing targeted tissues. This is known as ""therapeutic use.""Iodine-131 can be ""seen"" by nuclear medicine imaging techniques (i.e., gamma cameras) whenever it is given for therapeutic use, since about 10% of its energy and radiation dose is via gamma radiation. However, since the other 90% of radiation (beta radiation) causes tissue damage without contributing to any ability to see or ""image"" the isotope, other less-damaging radioisotopes of iodine such as iodine-123 (see isotopes of iodine) are preferred in situations when only nuclear imaging is required. The isotope I-131 is still occasionally used for purely diagnostic (i.e., imaging) work, due to its low expense compared to other iodine radioisotopes. Very small medical imaging doses of I-131 have not shown any increase in thyroid cancer. The low-cost availability of I-131, in turn, is due to the relative ease of creating I-131 by neutron bombardment of natural tellurium in a nuclear reactor, then separating I-131 out by various simple methods (i.e., heating to drive off the volatile iodine). By contrast, other iodine radioisotopes are usually created by far more expensive techniques, starting with reactor radiation of expensive capsules of pressurized xenon gas.Iodine-131 is also one of the most commonly used gamma-emitting radioactive industrial tracer. Radioactive tracer isotopes are injected with hydraulic fracturing fluid to determine the injection profile and location of fractures created by hydraulic fracturing.Much smaller incidental doses of iodine-131 than those used in medical therapeutic procedures, are thought to be the major cause of increased thyroid cancers after accidental nuclear contamination. These cancers happen from residual tissue radiation damage caused by the I-131, and usually appear years after exposure, long after the I-131 has decayed.