Goal 1 Study Guide and Practice Problems Fill in the following table
... 14. An electron configuration can be effective for describing the number of energy levels and the number of valence electrons for all of the elements. In the quantum mechanical model, what is the maximum number of electrons allowed in each of the first four energy levels? a. 1st energy level = 2 b. ...
... 14. An electron configuration can be effective for describing the number of energy levels and the number of valence electrons for all of the elements. In the quantum mechanical model, what is the maximum number of electrons allowed in each of the first four energy levels? a. 1st energy level = 2 b. ...
The Periodic Law
... 12. In the periodic table, the atomic masses of Te and I decrease rather than increase, while their atomic numbers increase. This phenomenon happens to other neighboring elements in the periodic table. Name two of these sets of elements. ...
... 12. In the periodic table, the atomic masses of Te and I decrease rather than increase, while their atomic numbers increase. This phenomenon happens to other neighboring elements in the periodic table. Name two of these sets of elements. ...
Chocolate Challenge - Waterford Public Schools
... Protons determine element’s identity # of protons is unique for each element Electrons determine element’s chemical properties Neutrons act as a “glue” for the protons to minimize charge repulsions ...
... Protons determine element’s identity # of protons is unique for each element Electrons determine element’s chemical properties Neutrons act as a “glue” for the protons to minimize charge repulsions ...
File
... A different amount of energy is required each time an e- is removed 1st ionization energy – remove 1st electron 2nd ionization energy – remove 2nd electron 3rd ionization energy – remove 3rd electron ...
... A different amount of energy is required each time an e- is removed 1st ionization energy – remove 1st electron 2nd ionization energy – remove 2nd electron 3rd ionization energy – remove 3rd electron ...
Chapter Two - Alfred State College intranet site
... Calculate the atomic mass of an element from the masses and abundances of its isotopes. Determine the number of atoms in a molecule from its chemical formula. Describe the arrangement of elements in the periodic table and explain the usefulness of the table. ...
... Calculate the atomic mass of an element from the masses and abundances of its isotopes. Determine the number of atoms in a molecule from its chemical formula. Describe the arrangement of elements in the periodic table and explain the usefulness of the table. ...
Looking for Patterns in Chemical Reactivity
... Elements and Compounds An element is a pure substance that cannon be broken down into simpler substances by ordinary means such as a chemical reaction. (Iron, Oxygen, and Carbon are all elements.) A compound is a pure substance made up of two or more elements that are chemically combined (Water, ...
... Elements and Compounds An element is a pure substance that cannon be broken down into simpler substances by ordinary means such as a chemical reaction. (Iron, Oxygen, and Carbon are all elements.) A compound is a pure substance made up of two or more elements that are chemically combined (Water, ...
Slide 1 - Images
... • Isotopes of an atom have the same number of protons but different numbers of electrons • Mass number is the number of neutrons plus the number of protons • Average atomic mass – the average mass of the mixture of an elements isotopes • The strong nuclear force holds tightly packed protons together ...
... • Isotopes of an atom have the same number of protons but different numbers of electrons • Mass number is the number of neutrons plus the number of protons • Average atomic mass – the average mass of the mixture of an elements isotopes • The strong nuclear force holds tightly packed protons together ...
CHEMISTRY FALL FINAL PRACTICE 2016
... a. Get the atomic number _________ b. Identify the element __________________ c. Find the mass number of the most common isotope of an element _________ d. Get how many neutrons the most common isotope has _________ e. How many valence electrons an element has _________ f. What is its charge/oxidati ...
... a. Get the atomic number _________ b. Identify the element __________________ c. Find the mass number of the most common isotope of an element _________ d. Get how many neutrons the most common isotope has _________ e. How many valence electrons an element has _________ f. What is its charge/oxidati ...
VL: 0
... 1. Complete RM 16 with your shoulder partner by placing Bohr models of 14 elements in the correct place on the modified Periodic Table. 2. Answer the questions on RM 18. 3. Complete RM 19. ...
... 1. Complete RM 16 with your shoulder partner by placing Bohr models of 14 elements in the correct place on the modified Periodic Table. 2. Answer the questions on RM 18. 3. Complete RM 19. ...
Atom and periodic table review
... Ductile – Able to be drawn into wires Malleable — Able to be hammered into sheets Metalloids – Seven elements which have properties of both metals and nonmetals. People: Moseley: English chemist (worked under Ernie Rutherford) who discovered the concept of atomic number. He refined the periodic tabl ...
... Ductile – Able to be drawn into wires Malleable — Able to be hammered into sheets Metalloids – Seven elements which have properties of both metals and nonmetals. People: Moseley: English chemist (worked under Ernie Rutherford) who discovered the concept of atomic number. He refined the periodic tabl ...
PowerPoint Presentation - The Atom: Chp 12 sect 2
... the same, • although the physical properties of some isotopes may be different. • Some isotopes are radioactivemeaning they "radiate" energy as they decay to a more stable form, • perhaps another element half-life: time required for half of the atoms of an element to decay into stable form. ...
... the same, • although the physical properties of some isotopes may be different. • Some isotopes are radioactivemeaning they "radiate" energy as they decay to a more stable form, • perhaps another element half-life: time required for half of the atoms of an element to decay into stable form. ...
5-1 Development of the Periodic Table
... Ionic Radii – the size of an ion. • Cation – a positive ion:Na+,Mg2+,Fe2+, Fe3+, – Formed by the loss of an electron – Cations are smaller than their neutral atom. • As an electron is lost, the effective nuclear charge is greater, causing the electron cloud to become pulled closer to the nucleus, m ...
... Ionic Radii – the size of an ion. • Cation – a positive ion:Na+,Mg2+,Fe2+, Fe3+, – Formed by the loss of an electron – Cations are smaller than their neutral atom. • As an electron is lost, the effective nuclear charge is greater, causing the electron cloud to become pulled closer to the nucleus, m ...
Chapter 3 pages 65
... Rutherford suggested that this bundle was the nucleus. He also suggested that the electrons orbited around the nucleus. This discovery was important because it revealed more about the atoms structure. ...
... Rutherford suggested that this bundle was the nucleus. He also suggested that the electrons orbited around the nucleus. This discovery was important because it revealed more about the atoms structure. ...
4. - period2chem
... There are small jumps in 1st ionization energy when there is an element with increased stability (full or half-full sublevel). Removing the 4th electron from aluminum represents removing a core electron. a. ionic, b. polar, c. ionic, d. nonpolar a. metallic, b. ionic, c. metallic, d. covalent, e. io ...
... There are small jumps in 1st ionization energy when there is an element with increased stability (full or half-full sublevel). Removing the 4th electron from aluminum represents removing a core electron. a. ionic, b. polar, c. ionic, d. nonpolar a. metallic, b. ionic, c. metallic, d. covalent, e. io ...
Atomic Theory
... Atomic number = number of protons in the nucleus Atomic number determines the identity of the element Mass number = protons + neutrons Number of electrons = number of protons Isotopes: two atoms of the same element with different numbers of neutrons C-12 and C-13 are isotopes of carbon ...
... Atomic number = number of protons in the nucleus Atomic number determines the identity of the element Mass number = protons + neutrons Number of electrons = number of protons Isotopes: two atoms of the same element with different numbers of neutrons C-12 and C-13 are isotopes of carbon ...
Periodicity of Elements
... 0 is the symbol for oxygen. and Ne is the symbol for neon. Why, then. isn't I or Ir the symbol for iron? Because scientists already had a name for iron-it was ferrum. They decided to use the already well-known Latin term for iron (and ten other elements). The exercises below will help you find and l ...
... 0 is the symbol for oxygen. and Ne is the symbol for neon. Why, then. isn't I or Ir the symbol for iron? Because scientists already had a name for iron-it was ferrum. They decided to use the already well-known Latin term for iron (and ten other elements). The exercises below will help you find and l ...
Pearson Prentice Hall Physical Science: Concepts in Action
... charged subatomic particle that is found in the nucleus of an atom Definition: an electron is a negatively charged subatomic particle found in the space OUTSIDE THE NUCLEUS ...
... charged subatomic particle that is found in the nucleus of an atom Definition: an electron is a negatively charged subatomic particle found in the space OUTSIDE THE NUCLEUS ...
4 1 introduction to atoms 65-68
... nucleus of an atom was ________________________. 7. In the atomic model proposed by ________________________ , electrons move in specific orbits, similar to how planets orbit the sun. 8. What particle did Chadwick discover in 1932 that was hard to detect because it had no electrical charge? ________ ...
... nucleus of an atom was ________________________. 7. In the atomic model proposed by ________________________ , electrons move in specific orbits, similar to how planets orbit the sun. 8. What particle did Chadwick discover in 1932 that was hard to detect because it had no electrical charge? ________ ...
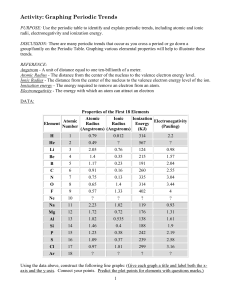
LSHS Graphing Periodic Trends Lab
... Ionization energy - The energy required to remove an electron from an atom. Electronegativity - The energy with which an atom can attract an electron ...
... Ionization energy - The energy required to remove an electron from an atom. Electronegativity - The energy with which an atom can attract an electron ...
Structure of the Atom - Saint Mary Catholic School
... • Atoms are the building blocks of matter • Atoms are too small in size to study easily • Size of Earth : soda can = soda can : atom ...
... • Atoms are the building blocks of matter • Atoms are too small in size to study easily • Size of Earth : soda can = soda can : atom ...
Document
... • Atoms are the building blocks of matter • Atoms are too small in size to study easily • Size of Earth : soda can = soda can : atom ...
... • Atoms are the building blocks of matter • Atoms are too small in size to study easily • Size of Earth : soda can = soda can : atom ...
Electron cloud model
... • Atoms are the building blocks of matter • Atoms are too small in size to study easily • Size of Earth : soda can = soda can : atom ...
... • Atoms are the building blocks of matter • Atoms are too small in size to study easily • Size of Earth : soda can = soda can : atom ...
Atomic Theory PPT
... Niels Bohr Niels Bohr stated that electrons move in different orbits, or energy levels, around the nucleus like planets orbit the sun. Each energy level is located a specific distance from the nucleus and contains a certain number of electrons. ...
... Niels Bohr Niels Bohr stated that electrons move in different orbits, or energy levels, around the nucleus like planets orbit the sun. Each energy level is located a specific distance from the nucleus and contains a certain number of electrons. ...