Assignment Chemistry Class XI (2016-17)
... 1. Express decimal equivalent of 2/3 to three significance places. 2. The human body temperature is 98.6 0 F. What is value in 0C and K? 3. One atom of an element weights is 9.75 ×10-23 g. calculate its atomic mass. 4.Round up the following to three significant figure (i) 10.4207 (ii) 0.04597 (iii) ...
... 1. Express decimal equivalent of 2/3 to three significance places. 2. The human body temperature is 98.6 0 F. What is value in 0C and K? 3. One atom of an element weights is 9.75 ×10-23 g. calculate its atomic mass. 4.Round up the following to three significant figure (i) 10.4207 (ii) 0.04597 (iii) ...
Dr. Ali Ebneshahidi © 2016 Ebneshahidi
... cells by changing their internal H2O volume. - Hypertonic: solutions with higher osmotic pressure cells in a Hypertonic solution lose H2O and shrink. - Hypotonic : solution with a lower osmotic pressure cells in hyportonic solution gain H2O and swell. - Isotonic : same tonicity - cell in isoto ...
... cells by changing their internal H2O volume. - Hypertonic: solutions with higher osmotic pressure cells in a Hypertonic solution lose H2O and shrink. - Hypotonic : solution with a lower osmotic pressure cells in hyportonic solution gain H2O and swell. - Isotonic : same tonicity - cell in isoto ...
Honors Chemistry Exam Review Questions
... is a pure substance has a variable composition can be physically separated into its elements has properties similar to those of its elements ...
... is a pure substance has a variable composition can be physically separated into its elements has properties similar to those of its elements ...
AP Chem Test 5-7 Practice Exam - mvhs
... 25.0 g of Na2O2 with water is __________ kJ. 2 Na2O2 (s) + 2 H2O(l) 4 NaOH(s) + O2(g) A) 20.2 B) 40.4 C) 67.5 D) 80.8 E) -126 2. The ΔH for the exothermic solution process when solid sodium hydroxide dissolves in water is 44.4 kJ/mol. When a 13.9-g sample of NaOH dissolves in 250.0 g of water in a ...
... 25.0 g of Na2O2 with water is __________ kJ. 2 Na2O2 (s) + 2 H2O(l) 4 NaOH(s) + O2(g) A) 20.2 B) 40.4 C) 67.5 D) 80.8 E) -126 2. The ΔH for the exothermic solution process when solid sodium hydroxide dissolves in water is 44.4 kJ/mol. When a 13.9-g sample of NaOH dissolves in 250.0 g of water in a ...
Energy Level Models - Middle School Chemistry
... substructure is made up of regions called orbitals which comprise each energy level. The shape and size of the orbital is defined by the space around the nucleus where there is a high probability of finding electrons. There can be a maximum of two electrons in any orbital so showing electrons in pai ...
... substructure is made up of regions called orbitals which comprise each energy level. The shape and size of the orbital is defined by the space around the nucleus where there is a high probability of finding electrons. There can be a maximum of two electrons in any orbital so showing electrons in pai ...
chemistry in the 8th grade
... chemical change is involved in changing physical state. A chemical change only occurs when chemical bonds are broken, formed, or both. When matter is in the solid state, the particles (atoms or molecules) that make up the matter are very close together. These particles can vibrate around fixed posit ...
... chemical change is involved in changing physical state. A chemical change only occurs when chemical bonds are broken, formed, or both. When matter is in the solid state, the particles (atoms or molecules) that make up the matter are very close together. These particles can vibrate around fixed posit ...
Fundamentals of Chemistry
... • The number of electrons in the valence shell determines the relative activity of an element. • The arrangement of electrons in the outer shell explains why some elements are chemically very active, some are not very active, and others are inert. • Group I has 1 valence electron, which makes it eas ...
... • The number of electrons in the valence shell determines the relative activity of an element. • The arrangement of electrons in the outer shell explains why some elements are chemically very active, some are not very active, and others are inert. • Group I has 1 valence electron, which makes it eas ...
Final Exam Review Day 1
... The Noble gases are stable because they have ___ ______________ ____________. Elements are placed in order of increasing ______________ _______________ and placed in groups according to their ___________________ __________________. Rows = ______________ =___________ __________ _______ Columns =_____ ...
... The Noble gases are stable because they have ___ ______________ ____________. Elements are placed in order of increasing ______________ _______________ and placed in groups according to their ___________________ __________________. Rows = ______________ =___________ __________ _______ Columns =_____ ...
the atomic theory
... 8. neutron 9. electron 10. shell 11. atomic number 12. atomic mass 13. Bohr Model 14. subatomic particle 15. isotope 16. empty bus seat rule B/ THE HISTORY OF THE ATOM: - John Dalton ...
... 8. neutron 9. electron 10. shell 11. atomic number 12. atomic mass 13. Bohr Model 14. subatomic particle 15. isotope 16. empty bus seat rule B/ THE HISTORY OF THE ATOM: - John Dalton ...
ď - Google Sites
... like” structure. He was the first to bring up the idea of an electron transfer due a chemical reaction to achieve this stability. Atoms would then be held together by having opposite charges. (Ionic bond) Gilbert Lewis (1916)- proposed that atoms could achieve a “noble gas like” stable structure by ...
... like” structure. He was the first to bring up the idea of an electron transfer due a chemical reaction to achieve this stability. Atoms would then be held together by having opposite charges. (Ionic bond) Gilbert Lewis (1916)- proposed that atoms could achieve a “noble gas like” stable structure by ...
1 Chemistry 400: General Chemistry Name: Miller Fall 2015 Final
... 4. There is a relationship between intermolecular forces, temperature, and the temperatures at which the solid, liquid, and gas phases exist. Each blank will be filled in with one of the words in bold. You may use each word more than once. (1 point per blank) A. ______________________ is proportion ...
... 4. There is a relationship between intermolecular forces, temperature, and the temperatures at which the solid, liquid, and gas phases exist. Each blank will be filled in with one of the words in bold. You may use each word more than once. (1 point per blank) A. ______________________ is proportion ...
Scientific Method - Virtual Medical Academy
... Chemistry:-The study of matter and the changes it can undergo MATTER:-Matter is any thing occupies space and has a mass. Matter------> has mass , mass to weight , occupies space. There are things you can see.. "e.x: water, tree, food". And there are things you can't see.. " air, gas in gas cylinders ...
... Chemistry:-The study of matter and the changes it can undergo MATTER:-Matter is any thing occupies space and has a mass. Matter------> has mass , mass to weight , occupies space. There are things you can see.. "e.x: water, tree, food". And there are things you can't see.. " air, gas in gas cylinders ...
Scientific Method - Virtual Medical Academy
... Chemistry:-The study of matter and the changes it can undergo MATTER:-Matter is any thing occupies space and has a mass. Matter------> has mass , mass to weight , occupies space. There are things you can see.. "e.x: water, tree, food". And there are things you can't see.. " air, gas in gas cylinders ...
... Chemistry:-The study of matter and the changes it can undergo MATTER:-Matter is any thing occupies space and has a mass. Matter------> has mass , mass to weight , occupies space. There are things you can see.. "e.x: water, tree, food". And there are things you can't see.. " air, gas in gas cylinders ...
Chapter 4.1 and 4.2 - science-b
... Atoms are composed of protons, neutrons, and electrons. Protons and neutrons are found in the tiny nucleus of the atom . The cloud that they form is the majority of the atom’s size. ...
... Atoms are composed of protons, neutrons, and electrons. Protons and neutrons are found in the tiny nucleus of the atom . The cloud that they form is the majority of the atom’s size. ...
S294 Are you Ready for S294 e1i1 web029856
... form ionic bonds with other atoms by transferring bonding electrons, and so themselves become positively charged ions. The atoms of the element to which the metal transfers electrons become negatively charged ions, and the resulting molecules are electrically neutral overall. Sodium chloride (common ...
... form ionic bonds with other atoms by transferring bonding electrons, and so themselves become positively charged ions. The atoms of the element to which the metal transfers electrons become negatively charged ions, and the resulting molecules are electrically neutral overall. Sodium chloride (common ...
ch14 lecture 7e
... Highlights of Boron Chemistry All boron compounds are covalent, and B forms a variety of network covalent compounds with other elements. Boron is often electron-deficient in compounds, and acts effectively as a Lewis acid since it can accept an e- pair. BF3(g) + :NH3(g) → F3B–NH3(g) Boron forms bri ...
... Highlights of Boron Chemistry All boron compounds are covalent, and B forms a variety of network covalent compounds with other elements. Boron is often electron-deficient in compounds, and acts effectively as a Lewis acid since it can accept an e- pair. BF3(g) + :NH3(g) → F3B–NH3(g) Boron forms bri ...
Atomic History Notes.notebook
... Dalton devised an atomic theory (early 1800's) based on the following points: 1) Elements are composed of extremely small and indivisible particles called atoms. 2) Atoms of the same element are identical. 3) Atoms combine chemically in simple whole number ratios, H2O is a 2:1 ratio of hydrogen and ...
... Dalton devised an atomic theory (early 1800's) based on the following points: 1) Elements are composed of extremely small and indivisible particles called atoms. 2) Atoms of the same element are identical. 3) Atoms combine chemically in simple whole number ratios, H2O is a 2:1 ratio of hydrogen and ...
Exam Review - hrsbstaff.ednet.ns.ca
... of an atom increases, will the ionization energy increase or decrease? 22. As the positive charge on an ion increases, will the ionization energy increase or decrease? 23. How are substances that are gases or soft solids at room temperature classified? 24. Would an element with two outer electrons b ...
... of an atom increases, will the ionization energy increase or decrease? 22. As the positive charge on an ion increases, will the ionization energy increase or decrease? 23. How are substances that are gases or soft solids at room temperature classified? 24. Would an element with two outer electrons b ...
CHAPTER 1 -Chemistry -Matter -Elements -Atoms
... 2) A solid white substance A is heated strongly in the absence of air. It decomposes to form a new white substance B and a gas C. The gas has exactly the same properties as the product obtained when carbon is burned in an excess of oxygen. Based on these observations, can you determine whether A and ...
... 2) A solid white substance A is heated strongly in the absence of air. It decomposes to form a new white substance B and a gas C. The gas has exactly the same properties as the product obtained when carbon is burned in an excess of oxygen. Based on these observations, can you determine whether A and ...
Year 9 Science revison _15-16_ end of year CHEM
... iv) The compound RbSO4 dissolves in water, but the element Rb doesn’t. Why ? The compound is made up of ions bonded together. When it dissolves in water, these ions separate from each other. They are charged (+ and -) and able to attract to and bond with water. This is why the ionic compound CAN dis ...
... iv) The compound RbSO4 dissolves in water, but the element Rb doesn’t. Why ? The compound is made up of ions bonded together. When it dissolves in water, these ions separate from each other. They are charged (+ and -) and able to attract to and bond with water. This is why the ionic compound CAN dis ...
Midterm Review 4
... 53. The ion with a charge of +1 and the same electron configuration as argon is a. potassium b. sodium c. neon d. magnesium 54. The tendency to lose electrons ______________ as we move across a period on the periodic table a. increases b. remains the same c. decreases d. no trend exists 55. Generall ...
... 53. The ion with a charge of +1 and the same electron configuration as argon is a. potassium b. sodium c. neon d. magnesium 54. The tendency to lose electrons ______________ as we move across a period on the periodic table a. increases b. remains the same c. decreases d. no trend exists 55. Generall ...
Name
... Essential Standard 6a: Carbon, because of its ability to combine in many ways with itself and other elements, has a central role in the chemistry of living organisms. ...
... Essential Standard 6a: Carbon, because of its ability to combine in many ways with itself and other elements, has a central role in the chemistry of living organisms. ...
AlBr3 E IO Ionic FU C O Cov Molec C IO Cov Molec Sn E N/A N/A
... combine in the ratio of small, whole numbers to form compounds. ...
... combine in the ratio of small, whole numbers to form compounds. ...
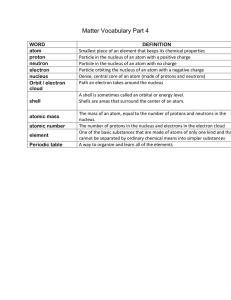
Matter Vocab Part 4
... Particle in the nucleus of an atom with a positive charge Particle in the nucleus of an atom with no charge Particle orbiting the nucleus of an atom with a negative charge Dense, central core of an atom (made of protons and neutrons) Path an electron takes around the nucleus A shell is sometimes cal ...
... Particle in the nucleus of an atom with a positive charge Particle in the nucleus of an atom with no charge Particle orbiting the nucleus of an atom with a negative charge Dense, central core of an atom (made of protons and neutrons) Path an electron takes around the nucleus A shell is sometimes cal ...