35 IChO Problems 1-13
... 2 was first observed experimentally in the early sixties. This means that although coulombic repulsion is important at short distances, covalent bonding must be very strong and, indeed, takes over stabilizing the system. A triple bond is formed by the coupling of the three unpaired p electrons on ea ...
... 2 was first observed experimentally in the early sixties. This means that although coulombic repulsion is important at short distances, covalent bonding must be very strong and, indeed, takes over stabilizing the system. A triple bond is formed by the coupling of the three unpaired p electrons on ea ...
13 CHEMICAL EQUILIBRIUM W MODULE - 5
... reaction, it is believed that all the reactants would be converted into products with the release or absorption of energy. This is not true in all cases. Many chemical reactions proceed only to a certain extent and stop. When analysed, the resulting mixture contains both the reactants and products. ...
... reaction, it is believed that all the reactants would be converted into products with the release or absorption of energy. This is not true in all cases. Many chemical reactions proceed only to a certain extent and stop. When analysed, the resulting mixture contains both the reactants and products. ...
OCR Gateway Science
... (a) Explain why solid calcium chloride does not conduct electricity. (b) What condition is needed to electrolyse pure calcium chloride? State the electrode products at the anode and cathode. (c) Predict the electrode products when a very dilute solution of calcium chloride in water is electrolysed. ...
... (a) Explain why solid calcium chloride does not conduct electricity. (b) What condition is needed to electrolyse pure calcium chloride? State the electrode products at the anode and cathode. (c) Predict the electrode products when a very dilute solution of calcium chloride in water is electrolysed. ...
Thermodynamics: Entropy and Free Energy
... + oxygen gas. Energy is conserved whether the process runs backward or forward, but there is an allowed direction in which these events always occur. In fact, most chemical and physical changes naturally occur in one direction and can occur in the opposite direction only with assistance. For example ...
... + oxygen gas. Energy is conserved whether the process runs backward or forward, but there is an allowed direction in which these events always occur. In fact, most chemical and physical changes naturally occur in one direction and can occur in the opposite direction only with assistance. For example ...
Principles of Chemistry 1 and 2 Notes
... - Molecular geometry is the 3-D arrangement of atoms in a molecule. - Molecular geometry affects the chemical and physical properties of the molecule (i.e. density, boiling points, melting points, etc.) - Molecular geometry is the geometry on the central atom related to terminal atoms. - The model w ...
... - Molecular geometry is the 3-D arrangement of atoms in a molecule. - Molecular geometry affects the chemical and physical properties of the molecule (i.e. density, boiling points, melting points, etc.) - Molecular geometry is the geometry on the central atom related to terminal atoms. - The model w ...
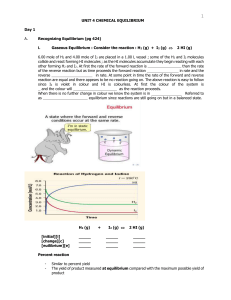
Unit 4 - Chemical Equilibrium
... Recognizing Equilibrium (pg 424) Gaseous Equilibrium : Consider the reaction : H2 (g) + I2 (g) ...
... Recognizing Equilibrium (pg 424) Gaseous Equilibrium : Consider the reaction : H2 (g) + I2 (g) ...
Line 4: Equation
... 6. Write the given information on the appropriate line above the equation. Lines 1 through 3 are your givens. Later, we will call these theoretical lines. In this example, we were given 2.50 mol of magnesium. Moles go on line 2. Write 2.50 above magnesium on line 2. See violet text in chart. 7. Calc ...
... 6. Write the given information on the appropriate line above the equation. Lines 1 through 3 are your givens. Later, we will call these theoretical lines. In this example, we were given 2.50 mol of magnesium. Moles go on line 2. Write 2.50 above magnesium on line 2. See violet text in chart. 7. Calc ...
Ionic Liquids Beyond Simple Solvents: Glimpses at the State of the
... 1990s,[1] ionic liquids (or “ILs”, for short) have attracted tremendous attention from virtually all major fields of chemistry.[2–6] Like all new “trendy” topics, ILs were expected to be the “solution” for all currently unsolved chemical problems. Soon, all kinds of experiments and synthetic procedu ...
... 1990s,[1] ionic liquids (or “ILs”, for short) have attracted tremendous attention from virtually all major fields of chemistry.[2–6] Like all new “trendy” topics, ILs were expected to be the “solution” for all currently unsolved chemical problems. Soon, all kinds of experiments and synthetic procedu ...
Chapter 6 Table of Contents
... At Contrived State University in Anytown, Ohio, a new building was dedicated in March 2010 to house the College of Education. The 100,000-square-foot building has enough office space to accommodate 86 full-time faculty members and 167 full-time staff. In a fit of monetary excess, the university admi ...
... At Contrived State University in Anytown, Ohio, a new building was dedicated in March 2010 to house the College of Education. The 100,000-square-foot building has enough office space to accommodate 86 full-time faculty members and 167 full-time staff. In a fit of monetary excess, the university admi ...
Chem. 1310 Fall 2005 Final Exam-white ... Name _________________________________ Section Number ___________________
... 21. Things that happen spontaneously a. increase the entropy of the universe. b. decrease the energy of the universe. Answer: a 22. Which of the following are generally true? a. Intermolecular forces are weaker than covalent bonds. b. Intermolecular forces are more directional than covalent bonds. c ...
... 21. Things that happen spontaneously a. increase the entropy of the universe. b. decrease the energy of the universe. Answer: a 22. Which of the following are generally true? a. Intermolecular forces are weaker than covalent bonds. b. Intermolecular forces are more directional than covalent bonds. c ...
Chemistry 120
... Solutions are normally liquid but solutions of gases in solids and solids in solids are known. ...
... Solutions are normally liquid but solutions of gases in solids and solids in solids are known. ...
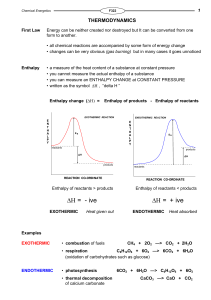
Enthalpy change
... Imagine that, during a reaction, all the bonds of reacting species are broken and the individual atoms join up again but in the form of products. The overall energy change will depend on the difference between the energy required to break the bonds and that released as bonds are made. energy release ...
... Imagine that, during a reaction, all the bonds of reacting species are broken and the individual atoms join up again but in the form of products. The overall energy change will depend on the difference between the energy required to break the bonds and that released as bonds are made. energy release ...
231. - Department of Chemistry
... with the ligands used in this study. The selected-ion flow tube (SIFT) technique was again used to take rate measurements since it is highly suitable for the investigation of the ligation of cations with weakly bonded ligands due to the relatively high helium pressure of the bath gas (0.35 Torr). Th ...
... with the ligands used in this study. The selected-ion flow tube (SIFT) technique was again used to take rate measurements since it is highly suitable for the investigation of the ligation of cations with weakly bonded ligands due to the relatively high helium pressure of the bath gas (0.35 Torr). Th ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.