summer learning G10
... 1. In the space below draw the Bohr diagram of the atomic structure (ie protons, neutrons and electrons). Label charge and relative mass of each sub-atomic particle. Help video: https://youtu.be/mBpnABBApu8 ...
... 1. In the space below draw the Bohr diagram of the atomic structure (ie protons, neutrons and electrons). Label charge and relative mass of each sub-atomic particle. Help video: https://youtu.be/mBpnABBApu8 ...
Quiz 1 - sample quiz
... 5. When a water solution of potassium sulfate is added to a water solution of strontium nitrate, a precipitate of strontium sulfate forms. What is the correctly balanced net ionic equation for the reaction? a) K2SO4(aq) + Sr(NO3)2(aq) SrSO4(s) + 2KNO3(aq) b) 2K+(aq) + SO42–(aq) + Sr2+(aq) + 2NO3–( ...
... 5. When a water solution of potassium sulfate is added to a water solution of strontium nitrate, a precipitate of strontium sulfate forms. What is the correctly balanced net ionic equation for the reaction? a) K2SO4(aq) + Sr(NO3)2(aq) SrSO4(s) + 2KNO3(aq) b) 2K+(aq) + SO42–(aq) + Sr2+(aq) + 2NO3–( ...
PowerPoint 演示文稿 - Shandong University
... The closed shell is spherically symmetric, and is strongly bound to the nucleus. The valence electron is located at a relatively large distance r from the nucleus. It moves in the electrostatic field of the nuclear charge +Ze, which is for the most part screened by the (Z-1) inner electrons. We desc ...
... The closed shell is spherically symmetric, and is strongly bound to the nucleus. The valence electron is located at a relatively large distance r from the nucleus. It moves in the electrostatic field of the nuclear charge +Ze, which is for the most part screened by the (Z-1) inner electrons. We desc ...
Lecture 5 Molecular Orbital Theory Part 1 Molecular Orbital Theory
... and mobile. Can you guess why? Hint does rotating about any of the carbon-carbon bonds break any orbital overlaps? ...
... and mobile. Can you guess why? Hint does rotating about any of the carbon-carbon bonds break any orbital overlaps? ...
Atoms and Ions
... Millikan determined the charge of an electron. He used an apparatus, as shown below, to produce tiny oil droplets. Very fine oil droplets were sprayed into a chamber and then were allowed to fall between two charged plates where they were then observed, visually. The air inside the chamber was expos ...
... Millikan determined the charge of an electron. He used an apparatus, as shown below, to produce tiny oil droplets. Very fine oil droplets were sprayed into a chamber and then were allowed to fall between two charged plates where they were then observed, visually. The air inside the chamber was expos ...
Chemistry basics powerpoint Chapter 2
... Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings ...
... Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings ...
ψ 2
... both be accommodated in the 1sg orbital if their spins are paired and the molecular orbital configuration for H2 is 1sg2. Since the 1sg orbital is the only occupied orbital in the ground state of H2, the density distribution shown previously in Fig. 62 for H2 is also the density distribution for the ...
... both be accommodated in the 1sg orbital if their spins are paired and the molecular orbital configuration for H2 is 1sg2. Since the 1sg orbital is the only occupied orbital in the ground state of H2, the density distribution shown previously in Fig. 62 for H2 is also the density distribution for the ...
3. Carbon nanostructures - Acclab h55.it.helsinki.fi
... - If you draw out the 4 bonds to equal length, then draw planes through the ends, you will get an ideal tetrahedron pyramid shape. The carbon bonds are very strong, typical energy/bond is of the order of 4 eV if they are to other carbon atoms. ...
... - If you draw out the 4 bonds to equal length, then draw planes through the ends, you will get an ideal tetrahedron pyramid shape. The carbon bonds are very strong, typical energy/bond is of the order of 4 eV if they are to other carbon atoms. ...
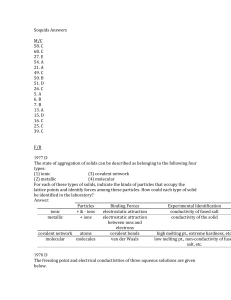
Soquids Answers M/C 58. C 68. C 27. E 54. A 21. A 49. C 50. B 51
... (ii) the amount of boiling point elevation depends on the number of non-volatile particles in solution. since the salt dissociates into 2 particles for every NaCl that dissolves, it will increase the boiling point more that an equal concentration of sugar (a molecular cpd) that does not dissociate o ...
... (ii) the amount of boiling point elevation depends on the number of non-volatile particles in solution. since the salt dissociates into 2 particles for every NaCl that dissolves, it will increase the boiling point more that an equal concentration of sugar (a molecular cpd) that does not dissociate o ...
Chemistry as a Game of Molecular Construction. The Bond-Click Way Brochure
... 8.2.6 Solubility and Insolubility of Ionic Materials 240 8.3 The Use of Ionic Matter in Living Organisms 242 8.3.1 Soluble Ionic Material Takes Care of Biological Communication 242 8.3.2 The Insoluble Ionic Material Makes Our Skeleton and Teeth 243 8.4 Covalent Molecules that Form Ions in Solution: ...
... 8.2.6 Solubility and Insolubility of Ionic Materials 240 8.3 The Use of Ionic Matter in Living Organisms 242 8.3.1 Soluble Ionic Material Takes Care of Biological Communication 242 8.3.2 The Insoluble Ionic Material Makes Our Skeleton and Teeth 243 8.4 Covalent Molecules that Form Ions in Solution: ...
1-Three states of matter . A: density, volume and weight B: solid
... Non-polar molecular crystals are very soft and are soluble in non-polar solvents. Non-polar molecular crystals are formed from symmetrical molecules with covalent bonds between atoms with small electronegativity differences. ...
... Non-polar molecular crystals are very soft and are soluble in non-polar solvents. Non-polar molecular crystals are formed from symmetrical molecules with covalent bonds between atoms with small electronegativity differences. ...
FINAL EXAM Review Sheet / Study Guide Honors Chemistry
... 36) How does the energy of an electron change when the electron moves closer to the nucleus? Farther away? ...
... 36) How does the energy of an electron change when the electron moves closer to the nucleus? Farther away? ...
Science 9 Unit 2
... the reaction. E.g. a sugar cube takes longer to dissolve than regular refined sugar Energy – the type of energy used will determine how fast the reaction occurs. E.g. if you use electrical energy from a battery the reaction will be faster ...
... the reaction. E.g. a sugar cube takes longer to dissolve than regular refined sugar Energy – the type of energy used will determine how fast the reaction occurs. E.g. if you use electrical energy from a battery the reaction will be faster ...
AP Chemistry Ch. 3 Sections 3.7-3.8 Notes Chemical Equations
... Bonds have been broken, and new ones have been formed. Important!!! In a chemical reaction, atoms are neither created nor destroyed. All atoms present in the reactants must be accounted for among the products. In other words, there must be the same number of each type of atom on the product side and ...
... Bonds have been broken, and new ones have been formed. Important!!! In a chemical reaction, atoms are neither created nor destroyed. All atoms present in the reactants must be accounted for among the products. In other words, there must be the same number of each type of atom on the product side and ...
Dr. Ali Ebneshahidi © 2016 Ebneshahidi
... atoms tend to change also – atoms that have either lost or gained electrons are called ions. Atoms that have lost electrons (as a result, now contain more p+ than e-) are called cations which carry positive charges, while atoms that have gained excessive electrons (as a result, now contain more etha ...
... atoms tend to change also – atoms that have either lost or gained electrons are called ions. Atoms that have lost electrons (as a result, now contain more p+ than e-) are called cations which carry positive charges, while atoms that have gained excessive electrons (as a result, now contain more etha ...
CH. 15 Notes
... to the right of the chemical symbols are called Subscripts, and notes the number of atoms of that element. A number in front of a chemical formula is a coefficient and it is multiplied by the subscript of all the atoms that are in the formula ...
... to the right of the chemical symbols are called Subscripts, and notes the number of atoms of that element. A number in front of a chemical formula is a coefficient and it is multiplied by the subscript of all the atoms that are in the formula ...
IPC Semester Exam Review – Chemistry Topics
... 59. List the subatomic particles & isotope symbol for bromine-80. 60. Calculate the average atomic mass of lithium if 1 of 13 atoms is lithium-6 & the other 12 atoms are lithium-7. Chemical Bonds 68. Why do most atoms form bonds to get 8 valence e ? Are these compounds ionic or covalent (# 69-71)? 6 ...
... 59. List the subatomic particles & isotope symbol for bromine-80. 60. Calculate the average atomic mass of lithium if 1 of 13 atoms is lithium-6 & the other 12 atoms are lithium-7. Chemical Bonds 68. Why do most atoms form bonds to get 8 valence e ? Are these compounds ionic or covalent (# 69-71)? 6 ...
Molecular Geometry and Polarity1
... even though the substances are comprised of a limited number of elements. Indeed, only a very small number of different elements are present in almost any pure substance we encounter in the environment or the laboratory. How can this wide diversity of properties be explained? A key to understanding ...
... even though the substances are comprised of a limited number of elements. Indeed, only a very small number of different elements are present in almost any pure substance we encounter in the environment or the laboratory. How can this wide diversity of properties be explained? A key to understanding ...
Exam #: Printed Name: Signature: PHYSICS
... Consider a simple model of the atomic nucleus as a cubical box with zero potential inside the box and infinite potential outside. The nucleons are thus confined to the box. In the model, they do not interact with each other: the strong interactions are modelled as producing the box potential. The leng ...
... Consider a simple model of the atomic nucleus as a cubical box with zero potential inside the box and infinite potential outside. The nucleons are thus confined to the box. In the model, they do not interact with each other: the strong interactions are modelled as producing the box potential. The leng ...
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electrostatic force of attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction. The strength of chemical bonds varies considerably; there are ""strong bonds"" such as covalent or ionic bonds and ""weak bonds"" such as Dipole-dipole interaction, the London dispersion force and hydrogen bonding.Since opposite charges attract via a simple electromagnetic force, the negatively charged electrons that are orbiting the nucleus and the positively charged protons in the nucleus attract each other. An electron positioned between two nuclei will be attracted to both of them, and the nuclei will be attracted toward electrons in this position. This attraction constitutes the chemical bond. Due to the matter wave nature of electrons and their smaller mass, they must occupy a much larger amount of volume compared with the nuclei, and this volume occupied by the electrons keeps the atomic nuclei relatively far apart, as compared with the size of the nuclei themselves. This phenomenon limits the distance between nuclei and atoms in a bond.In general, strong chemical bonding is associated with the sharing or transfer of electrons between the participating atoms. The atoms in molecules, crystals, metals and diatomic gases—indeed most of the physical environment around us—are held together by chemical bonds, which dictate the structure and the bulk properties of matter.All bonds can be explained by quantum theory, but, in practice, simplification rules allow chemists to predict the strength, directionality, and polarity of bonds. The octet rule and VSEPR theory are two examples. More sophisticated theories are valence bond theory which includes orbital hybridization and resonance, and the linear combination of atomic orbitals molecular orbital method which includes ligand field theory. Electrostatics are used to describe bond polarities and the effects they have on chemical substances.