Atomic Theory Notes Packet
... d block: Groups _____ through _____ filling (includes the ____________ elements) f block: _______________ and _______________ Series filling 2. Belated filling: when an ____ sublevel fills before a lower p sublevel. Example: 4s fills before the _____ 3. Valence Electrons: electrons in the __________ ...
... d block: Groups _____ through _____ filling (includes the ____________ elements) f block: _______________ and _______________ Series filling 2. Belated filling: when an ____ sublevel fills before a lower p sublevel. Example: 4s fills before the _____ 3. Valence Electrons: electrons in the __________ ...
Summaries of Review Topics for AP Chemistry
... Rule #1: Identify and name acids: acids are covalent compounds which formulas start with H (except H2O and H2O2). Find their name in the “Names and Formulas of Acids” below. If the acid is made with a polyatomic ion, change the ending of the ion from –ate to –ic, or from –ite to –ous and add acid to ...
... Rule #1: Identify and name acids: acids are covalent compounds which formulas start with H (except H2O and H2O2). Find their name in the “Names and Formulas of Acids” below. If the acid is made with a polyatomic ion, change the ending of the ion from –ate to –ic, or from –ite to –ous and add acid to ...
Appendix. Atoms and Molecule
... atom and is more stabile. The ionisation of takes of 5.1 eV but if we let the Na-electron join the Cl- ion we gain 3.8 eV, why the difference only will be 5.1 – 3.8 eV = 1.3 eV. We have thus formed a diatomic molecule with less energy than the energies of the two separate atoms, why it is stabile. 1 ...
... atom and is more stabile. The ionisation of takes of 5.1 eV but if we let the Na-electron join the Cl- ion we gain 3.8 eV, why the difference only will be 5.1 – 3.8 eV = 1.3 eV. We have thus formed a diatomic molecule with less energy than the energies of the two separate atoms, why it is stabile. 1 ...
LN_atoms_etc
... Modern View of Atomic Structure Experiments by Thomson and Millikan confirmed the existence of electrons as the negatively charged particles within an atom. Electrons have a charge of e = 1.6021773 10–19 C and a mass of 9.109390 10–31 kg. Later experiments by Rutherford determined that at the ce ...
... Modern View of Atomic Structure Experiments by Thomson and Millikan confirmed the existence of electrons as the negatively charged particles within an atom. Electrons have a charge of e = 1.6021773 10–19 C and a mass of 9.109390 10–31 kg. Later experiments by Rutherford determined that at the ce ...
Document
... (a) The anion from which this acid is derived is CN–, the cyanide ion. Because this ion has an -ide ending, the acid is given a hydro- prefix and an -ic ending: hydrocyanic acid. Only water solutions of HCN are referred to as hydrocyanic acid. The pure compound, which is a gas under normal condition ...
... (a) The anion from which this acid is derived is CN–, the cyanide ion. Because this ion has an -ide ending, the acid is given a hydro- prefix and an -ic ending: hydrocyanic acid. Only water solutions of HCN are referred to as hydrocyanic acid. The pure compound, which is a gas under normal condition ...
PowerPoint 演示文稿
... If we have a hydrogen atom with its electron in an excited state (either by light absorption or by heating) the electron may fall down to a lower orbit by emission of light. The electron may fall into any lower orbit, and the energy it loses will be exactly equal to the energy difference between the ...
... If we have a hydrogen atom with its electron in an excited state (either by light absorption or by heating) the electron may fall down to a lower orbit by emission of light. The electron may fall into any lower orbit, and the energy it loses will be exactly equal to the energy difference between the ...
Chapter 1 - Solutions
... unpaired electron spins. Identify each atom as diamagnetic or paramagnetic. a) N 1s2 2s2 2p3 = [He] 2s2 2p3 2 core electrons, 5 valence electrons 3 unpaired electron spins, paramagnetic b) Ca 1s2 2s2 2p6 3s2 3p6 4s2 = [Ar] 4s2 18 core electrons, 2 valence electrons 0 unpaired electron spins, diamagn ...
... unpaired electron spins. Identify each atom as diamagnetic or paramagnetic. a) N 1s2 2s2 2p3 = [He] 2s2 2p3 2 core electrons, 5 valence electrons 3 unpaired electron spins, paramagnetic b) Ca 1s2 2s2 2p6 3s2 3p6 4s2 = [Ar] 4s2 18 core electrons, 2 valence electrons 0 unpaired electron spins, diamagn ...
- skv institute
... In the crystal structure of ionic compounds, there is regular three dimensional arrangements of positive ions and negative ions. They are also combined with each other by Coulombic attraction forces. Such an arrangement or structure is also called crystal structure. 6 What is lattice enthalpy? T ...
... In the crystal structure of ionic compounds, there is regular three dimensional arrangements of positive ions and negative ions. They are also combined with each other by Coulombic attraction forces. Such an arrangement or structure is also called crystal structure. 6 What is lattice enthalpy? T ...
STRUCTURE OF A TURE OF A TURE OF ATOM STRUCTURE OF A
... 51. What is photoelectric effect? State the result of photoelectric effect experiment that could not be explained on the basis of laws of classical physics. Explain this effect on the basis of quantum theory of electromagnetic radiations. 52. Threshold frequency, ν0 is the minimum frequency which a ...
... 51. What is photoelectric effect? State the result of photoelectric effect experiment that could not be explained on the basis of laws of classical physics. Explain this effect on the basis of quantum theory of electromagnetic radiations. 52. Threshold frequency, ν0 is the minimum frequency which a ...
Chapter 14: Phenomena Chapter 14 Covalent Bonding: Orbitals
... Phenomena: Scientists knew that in order to form a bond, orbitals on two atoms must overlap. However, px, py, and pz orbitals are located 90˚ from each other and compounds like CH4 (which would form bonds using their p orbitals) do not have bond angles of 90˚. Therefore, scientists had to explain th ...
... Phenomena: Scientists knew that in order to form a bond, orbitals on two atoms must overlap. However, px, py, and pz orbitals are located 90˚ from each other and compounds like CH4 (which would form bonds using their p orbitals) do not have bond angles of 90˚. Therefore, scientists had to explain th ...
Chemistry Academic v. 2016
... Dalton’s Atomic Theory- Atomic theory – the belief that all matter is composed of tiny, indivisible elements; Isotope- An atom that has the same number of protons as other atoms of the same element do but that has different number of neutrons; Law of Conservation of Mass- Mass cannot be created or d ...
... Dalton’s Atomic Theory- Atomic theory – the belief that all matter is composed of tiny, indivisible elements; Isotope- An atom that has the same number of protons as other atoms of the same element do but that has different number of neutrons; Law of Conservation of Mass- Mass cannot be created or d ...
EM SPECTRUM, WAVELENGTH, FREQUENCY, AND ENERGY
... 11. Electrons give off energy in the form of a ____________________ when returning to the ground state. 12. Which scientist proposed the idea that electrons travel around the nucleus in fixed paths? 13. When an electron moves from the ground state to the excited state, energy is ____________________ ...
... 11. Electrons give off energy in the form of a ____________________ when returning to the ground state. 12. Which scientist proposed the idea that electrons travel around the nucleus in fixed paths? 13. When an electron moves from the ground state to the excited state, energy is ____________________ ...
Answers to Selected Questions and Problems
... (f) mass number; (g) isotope; (h) cation; (i) subatomic particle; (j) alkali metal; (k) periodic table Dalton used the laws of conservation of mass (Lavoisier) and definite proportions (Proust). They differ in their atomic masses and chemical properties. Compounds contain discrete numbers of atoms o ...
... (f) mass number; (g) isotope; (h) cation; (i) subatomic particle; (j) alkali metal; (k) periodic table Dalton used the laws of conservation of mass (Lavoisier) and definite proportions (Proust). They differ in their atomic masses and chemical properties. Compounds contain discrete numbers of atoms o ...
ELECTRONIC STRUCTURE OF THE ATOM
... The SPIN QUANTUM NUMBER, ms, represents electron spin. Since there are only two possible spins —- clockwise and counterclockwise — for an electron, ms can have two values: ─½ or +½. The spin quantum number led to the PAULI'S EXCLUSION PRINCIPLE. In a given atom, no two electrons can have the same se ...
... The SPIN QUANTUM NUMBER, ms, represents electron spin. Since there are only two possible spins —- clockwise and counterclockwise — for an electron, ms can have two values: ─½ or +½. The spin quantum number led to the PAULI'S EXCLUSION PRINCIPLE. In a given atom, no two electrons can have the same se ...
CLASS NOTES- Balancing Chemical Equations.pptx
... the right side for EACH element in order to balance the equation 4. Check your answer to see if: • The numbers of atoms on both sides of the equation are now balanced • The coefficients are in the lowest possible whole number ratios. (reduced) ...
... the right side for EACH element in order to balance the equation 4. Check your answer to see if: • The numbers of atoms on both sides of the equation are now balanced • The coefficients are in the lowest possible whole number ratios. (reduced) ...
Reactions and Equations
... How to Remember the Diatomic Elements • The elements ending with "-gen" including halogens form diatomic molecules. An easy-to-remember mnemonic for the diatomic elements is: Have No Fear Of Ice Cold Beverages ...
... How to Remember the Diatomic Elements • The elements ending with "-gen" including halogens form diatomic molecules. An easy-to-remember mnemonic for the diatomic elements is: Have No Fear Of Ice Cold Beverages ...
13.2 Chemical Formulas
... What is a chemical formula? Chemical formulas have two important parts: chemical symbols for the elements in the compound and subscripts that tell how many atoms of each element are needed to form the compound. The chemical formula for water, H2O, tells us that a water molecule is made of the elemen ...
... What is a chemical formula? Chemical formulas have two important parts: chemical symbols for the elements in the compound and subscripts that tell how many atoms of each element are needed to form the compound. The chemical formula for water, H2O, tells us that a water molecule is made of the elemen ...
File
... 3 = prop 7 = hept 4 = but 8 = oct Suffix is determined by the type of bond Alkane CnH2n+2 (all bonds are single) Alkene CnH2n (one bond is a double) Alkyne CnH2n-2 (one bond is a triple) ...
... 3 = prop 7 = hept 4 = but 8 = oct Suffix is determined by the type of bond Alkane CnH2n+2 (all bonds are single) Alkene CnH2n (one bond is a double) Alkyne CnH2n-2 (one bond is a triple) ...
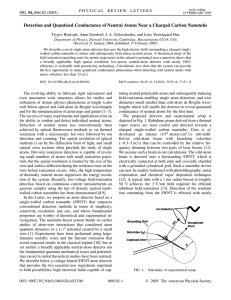
Detection and Quantized Conductance of Neutral Atoms Near a Charged... Trygve Ristroph, Anne Goodsell, J. A. Golovchenko, and Lene Vestergaard...
... The evolving ability to fabricate rigid micrometer and even nanometer scale structures allows for studies and utilization of atomic physics phenomena at length scales well below optical and cold-atom de Broglie wavelengths and for the miniaturization of atom traps and guides [1–7]. The success of ma ...
... The evolving ability to fabricate rigid micrometer and even nanometer scale structures allows for studies and utilization of atomic physics phenomena at length scales well below optical and cold-atom de Broglie wavelengths and for the miniaturization of atom traps and guides [1–7]. The success of ma ...
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electrostatic force of attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction. The strength of chemical bonds varies considerably; there are ""strong bonds"" such as covalent or ionic bonds and ""weak bonds"" such as Dipole-dipole interaction, the London dispersion force and hydrogen bonding.Since opposite charges attract via a simple electromagnetic force, the negatively charged electrons that are orbiting the nucleus and the positively charged protons in the nucleus attract each other. An electron positioned between two nuclei will be attracted to both of them, and the nuclei will be attracted toward electrons in this position. This attraction constitutes the chemical bond. Due to the matter wave nature of electrons and their smaller mass, they must occupy a much larger amount of volume compared with the nuclei, and this volume occupied by the electrons keeps the atomic nuclei relatively far apart, as compared with the size of the nuclei themselves. This phenomenon limits the distance between nuclei and atoms in a bond.In general, strong chemical bonding is associated with the sharing or transfer of electrons between the participating atoms. The atoms in molecules, crystals, metals and diatomic gases—indeed most of the physical environment around us—are held together by chemical bonds, which dictate the structure and the bulk properties of matter.All bonds can be explained by quantum theory, but, in practice, simplification rules allow chemists to predict the strength, directionality, and polarity of bonds. The octet rule and VSEPR theory are two examples. More sophisticated theories are valence bond theory which includes orbital hybridization and resonance, and the linear combination of atomic orbitals molecular orbital method which includes ligand field theory. Electrostatics are used to describe bond polarities and the effects they have on chemical substances.