Picobiology
... Picobiology is a field of biology in which we identify the protein(s) that drive physiologically important phenomenon, determine the structure at a resolution of 1 pm and elucidate the reaction catalyzed by the protein(s) with chemistry words. Primary research techniques include protein crystallogra ...
... Picobiology is a field of biology in which we identify the protein(s) that drive physiologically important phenomenon, determine the structure at a resolution of 1 pm and elucidate the reaction catalyzed by the protein(s) with chemistry words. Primary research techniques include protein crystallogra ...
Teacher Demo/Student Activity: Elephant`s Toothpaste
... example, a glowing splint placed near the foam should glow brighter due to the presence of oxygen. Challenge students to write the word and chemical equations for this reaction. This demonstration/activity could also be used to introduce some concepts that will be learned in senior level chemistry c ...
... example, a glowing splint placed near the foam should glow brighter due to the presence of oxygen. Challenge students to write the word and chemical equations for this reaction. This demonstration/activity could also be used to introduce some concepts that will be learned in senior level chemistry c ...
EXAM 3
... A 5.000 g sample of a compound known to contain only the elements phosphorous and oxygen was analyzed and found to contain 2.182 g of phosphorous. Additional experiments indicate that this compound has a molecular weight of 283.9 g/mol. How many phosphorous atoms are present in each molecule of this ...
... A 5.000 g sample of a compound known to contain only the elements phosphorous and oxygen was analyzed and found to contain 2.182 g of phosphorous. Additional experiments indicate that this compound has a molecular weight of 283.9 g/mol. How many phosphorous atoms are present in each molecule of this ...
Introductory Chemistry Test Review
... 9. For the following chemical compounds, predict whether each will be soluble or insoluble in aqueous solution. a. Al(OH)3 b. Hg2Cl2 c. (NH4)2CO3 10. For the following aqueous chemical reactions, predict the possible products and identify any products that will be insoluble. a. CaCl2 + K2S b. MgCl2 ...
... 9. For the following chemical compounds, predict whether each will be soluble or insoluble in aqueous solution. a. Al(OH)3 b. Hg2Cl2 c. (NH4)2CO3 10. For the following aqueous chemical reactions, predict the possible products and identify any products that will be insoluble. a. CaCl2 + K2S b. MgCl2 ...
Sample Questions
... C. What information are you looking for? D. What information do they give? E. How will you go about solving this? F. Show how to solve the problem. G. Be able to answer for a different reaction, number, set of conditions etc. ...
... C. What information are you looking for? D. What information do they give? E. How will you go about solving this? F. Show how to solve the problem. G. Be able to answer for a different reaction, number, set of conditions etc. ...
DALTON`S ATOMIC THEORY - 1808: Publication of Dalton`s "A New
... - Dalton's theory sets LIMITS on what can be done with chemistry. For example: Chemistry can't convert lead (an element) into gold (another element). Sorry, alchemists! You can't have a compound form in a chemical reaction that contains an element that was not in your starting materials. You can onl ...
... - Dalton's theory sets LIMITS on what can be done with chemistry. For example: Chemistry can't convert lead (an element) into gold (another element). Sorry, alchemists! You can't have a compound form in a chemical reaction that contains an element that was not in your starting materials. You can onl ...
Lecture 7. Fundamentals of atmospheric chemistry: Part 2 1
... (endothermic reactions), and a decrease in temperature favors the process that releases the heat (exothermic reactions). For the reaction above, the forward reaction releases the heat, and the reverse reaction absorbs heat. Therefore, the production of ammonia is favored by lowering T, because this ...
... (endothermic reactions), and a decrease in temperature favors the process that releases the heat (exothermic reactions). For the reaction above, the forward reaction releases the heat, and the reverse reaction absorbs heat. Therefore, the production of ammonia is favored by lowering T, because this ...
Solved Guess Paper – 3 Q1. Define the term molarity . Ans
... 11 a. Mention one consequence of metal excess defect . b. Give an example for molecular solid . Ans- a. Metal excess defect is due to anionic vacancies and extra contains at interstitial site . The anionic sites are occupied by unpaired electrons are called F- centres which imparts colour to the cry ...
... 11 a. Mention one consequence of metal excess defect . b. Give an example for molecular solid . Ans- a. Metal excess defect is due to anionic vacancies and extra contains at interstitial site . The anionic sites are occupied by unpaired electrons are called F- centres which imparts colour to the cry ...
Review Packet
... 28. Hugh was born 6.391875 X 103 days ago. How old (in years, with 1yr= 365.25 days) is Hugh? ...
... 28. Hugh was born 6.391875 X 103 days ago. How old (in years, with 1yr= 365.25 days) is Hugh? ...
Simple Chemical Reactions
... N4 Chemical change & structure - Energy changes of chemical reactions N4 Nature's Chemistry - Fuels N5 Nature's Chemistry - Energy from Fuels Revised Higher - Consumer Chemistry - 1c) Uses of alcohols ...
... N4 Chemical change & structure - Energy changes of chemical reactions N4 Nature's Chemistry - Fuels N5 Nature's Chemistry - Energy from Fuels Revised Higher - Consumer Chemistry - 1c) Uses of alcohols ...
astrochemistry_caselli
... 4. Formation and destructio1n of CO [a] C + H3O+ HCO+ + H2 [b] O + CH3+ HCO+ + H2 [c] HCO+ + e CO + H is the most important source of CO. CO is very stable and difficult to remove. It reacts with H3+: [d] H3+ + CO HCO+ + H2 but reaction [c] immediately reform CO. The main mechanisms for rem ...
... 4. Formation and destructio1n of CO [a] C + H3O+ HCO+ + H2 [b] O + CH3+ HCO+ + H2 [c] HCO+ + e CO + H is the most important source of CO. CO is very stable and difficult to remove. It reacts with H3+: [d] H3+ + CO HCO+ + H2 but reaction [c] immediately reform CO. The main mechanisms for rem ...
SAMPLE QUESTION PAPER CHEMISTRY (043) CLASS XII (2013-14)
... 5. Correct double helix structure of DNA 6. Which depends on the pore structure and the shape of reactants and products ...
... 5. Correct double helix structure of DNA 6. Which depends on the pore structure and the shape of reactants and products ...
Sample Paper Chemistry - Educomp Solutions Ltd.
... stability of phenoxide ion. The carboxylate ion is much more resonance stabilized than phenoxide ion. (ii) Semicarbazide has two –NH2 groups. One of them, which is directly attached to C=O is involved in resonance. Thus electron density on this group decreases and it does not act as a nucleophile. I ...
... stability of phenoxide ion. The carboxylate ion is much more resonance stabilized than phenoxide ion. (ii) Semicarbazide has two –NH2 groups. One of them, which is directly attached to C=O is involved in resonance. Thus electron density on this group decreases and it does not act as a nucleophile. I ...
4 - Ms McRae`s Science
... reaction) the reaction takes longer indicating a lower rate of reaction and must be due to the nature of the reactants. Surface area is not a factor as the reactants are all aqueous solutions. No catalyst is indicated. ...
... reaction) the reaction takes longer indicating a lower rate of reaction and must be due to the nature of the reactants. Surface area is not a factor as the reactants are all aqueous solutions. No catalyst is indicated. ...
Chemical reactions
... • Ionic - lacking discrete unit, or molecule • Composed of both metallic and nonmetallic elements • Electronegativity difference > 1.7 ...
... • Ionic - lacking discrete unit, or molecule • Composed of both metallic and nonmetallic elements • Electronegativity difference > 1.7 ...
Final Exam review semester 1
... For the chemical reaction C2H6 + 137 kJ → C2H4 + H2, the chemical energy of the ...
... For the chemical reaction C2H6 + 137 kJ → C2H4 + H2, the chemical energy of the ...
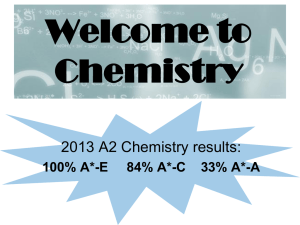
Welcome to Chemistry
... - Amounts of substance e.g. molecular formula, empirical formula, reacting mass ...
... - Amounts of substance e.g. molecular formula, empirical formula, reacting mass ...
DEPARTMENT OF CHEMISTRY
... a. Write the reactions (total of 5) for each of the secondary, tertiary, and aryl substrates listed in 1.e. above with ethanol and silver nitrate in the table on the next page. b. Obtain 5 clean, dry, new test tubes (10 x 75 mm size) and parafilm. Devise a scheme to enable you to keep track of each ...
... a. Write the reactions (total of 5) for each of the secondary, tertiary, and aryl substrates listed in 1.e. above with ethanol and silver nitrate in the table on the next page. b. Obtain 5 clean, dry, new test tubes (10 x 75 mm size) and parafilm. Devise a scheme to enable you to keep track of each ...