
Exam Review
... 37. The nuclide symbol 8 O represents an oxygen atom with a) a mass of 8 u c) a mass of 16 u b) an atomic number of 16 d) 16 neutrons ...
... 37. The nuclide symbol 8 O represents an oxygen atom with a) a mass of 8 u c) a mass of 16 u b) an atomic number of 16 d) 16 neutrons ...
File
... commonly thought of as a metal, does have some nonmetallic properties as its bonds to other nonmetals have significant covalent character. The other Group 3A elements have typical metal characteristics; its compounds formed with nonmetals are ionic. From this discussion, metallic character increases ...
... commonly thought of as a metal, does have some nonmetallic properties as its bonds to other nonmetals have significant covalent character. The other Group 3A elements have typical metal characteristics; its compounds formed with nonmetals are ionic. From this discussion, metallic character increases ...
Chemistry HL Syllabus Details
... A pair of electrons can be represented by dots, crosses, a combination of dots and crosses or by a line. For example, chlorine can be shown as: Cl ...
... A pair of electrons can be represented by dots, crosses, a combination of dots and crosses or by a line. For example, chlorine can be shown as: Cl ...
M - coercingmolecules
... by counting or weighing them, depending on which method is more convenient ...
... by counting or weighing them, depending on which method is more convenient ...
Chapter 03 - KFUPM Faculty List
... On the left hand side we have 1 O atom in ethanol, so we must provide 6 O atoms from O2 and thus we must have 3 O2, so the balanced equation is C2H5OH + 3 O2 2 CO2 + 3 H2O: 2C + 6H + 7O both on the left and on the right, so it is ok. (NH4)2Cr2O7 Cr2O3 + H2O + N2 left: 7O + 2Cr + 8H + 2N; right: ...
... On the left hand side we have 1 O atom in ethanol, so we must provide 6 O atoms from O2 and thus we must have 3 O2, so the balanced equation is C2H5OH + 3 O2 2 CO2 + 3 H2O: 2C + 6H + 7O both on the left and on the right, so it is ok. (NH4)2Cr2O7 Cr2O3 + H2O + N2 left: 7O + 2Cr + 8H + 2N; right: ...
chemistry-resource
... Students’ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials pre ...
... Students’ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials pre ...
study material(2014-15) class xii-chemistry
... Students‘ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials pre ...
... Students‘ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials pre ...
Calculations with Chemical Formulas and Equations
... The trick: • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
... The trick: • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
Stoichiometry - coercingmolecules
... by counting or weighing them, depending on which method is more convenient ...
... by counting or weighing them, depending on which method is more convenient ...
Final Exam
... ____ 23. What is the mole fraction of 1.98 m Fe(NO3)3(aq)? The molar mass of Fe(NO3)3 is 241.9 g/mol and the molar mass of water is 18.02 g/mol. a. 0.0345 b. 0.0641 c. 0.324 d. 0.479 e. 0.863 ____ 24. The vapor pressure of pure water at 45 C is 71.9 mm Hg. What is the vapor pressure of a mixture of ...
... ____ 23. What is the mole fraction of 1.98 m Fe(NO3)3(aq)? The molar mass of Fe(NO3)3 is 241.9 g/mol and the molar mass of water is 18.02 g/mol. a. 0.0345 b. 0.0641 c. 0.324 d. 0.479 e. 0.863 ____ 24. The vapor pressure of pure water at 45 C is 71.9 mm Hg. What is the vapor pressure of a mixture of ...
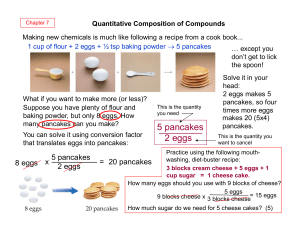
5 pancakes 2 eggs
... many grams? How many P atoms? How many O atoms? How many total atoms? 26. A 7.52 g sample of ajoene (garlic odor) was found to contain 3.09 g S, 0.453 g H, 0.513 g O and the rest, C. Calculate the percent composition. 39. Ethanedioic acid, a compound that is present in many vegetables, has a molar m ...
... many grams? How many P atoms? How many O atoms? How many total atoms? 26. A 7.52 g sample of ajoene (garlic odor) was found to contain 3.09 g S, 0.453 g H, 0.513 g O and the rest, C. Calculate the percent composition. 39. Ethanedioic acid, a compound that is present in many vegetables, has a molar m ...
To do List
... The element samarium is known to have three isotopes - Sm-148, Sm-149, and Sm-152. The masses of these three isotopes are 148.1010 amu, 149.2005 amu, and 152.4107 amu, respectively. If the lightest isotope is three times as abundant as the heaviest, and the middle isotope is known to be 16.00% abund ...
... The element samarium is known to have three isotopes - Sm-148, Sm-149, and Sm-152. The masses of these three isotopes are 148.1010 amu, 149.2005 amu, and 152.4107 amu, respectively. If the lightest isotope is three times as abundant as the heaviest, and the middle isotope is known to be 16.00% abund ...
PC_Chemistry_Macomb_April08
... Solids can be classified as metallic, ionic, covalent, or network covalent. These different types of solids have different properties that depend on the particles and forces found in the solid. Compare the relative strengths of forces between ...
... Solids can be classified as metallic, ionic, covalent, or network covalent. These different types of solids have different properties that depend on the particles and forces found in the solid. Compare the relative strengths of forces between ...
ab initio calculations of mechanical, thermodynamic and
... 9Al2 O3 • SiO2 , and ι-Al2 O3 (iota-alumina) are constructed starting from experimentally reported crystal structures. A large number of models were built for each phase and relaxed using the Vienna ab initio simulation package (VASP) program. The model with the lowest total energy for a given x was ...
... 9Al2 O3 • SiO2 , and ι-Al2 O3 (iota-alumina) are constructed starting from experimentally reported crystal structures. A large number of models were built for each phase and relaxed using the Vienna ab initio simulation package (VASP) program. The model with the lowest total energy for a given x was ...
DOE Chemistry 1
... their reactor-specific content, DOE Category A reactor training managers also reviewed and commented on the content. On the basis of feedback from these sources, information that applied to two or more DOE nuclear facilities was considered generic and was included. The final draft of each of the han ...
... their reactor-specific content, DOE Category A reactor training managers also reviewed and commented on the content. On the basis of feedback from these sources, information that applied to two or more DOE nuclear facilities was considered generic and was included. The final draft of each of the han ...
Exam Review Packet Table of Contents
... strike the particle. The photon has an energy comparable to that of an electron but small compared to that of a macroscopic object. They must stress mass rather than size as the important distinction ...
... strike the particle. The photon has an energy comparable to that of an electron but small compared to that of a macroscopic object. They must stress mass rather than size as the important distinction ...
New polyanion-based cathode materials for alkali
... Li3 Fe2(HPO3)3Cl, LiFe(HPO3)2, Li0.8 Fe(H2O)2B[P2O8]•H2O and AFePO4NO3 (A = NH4/Li, K). Furthermore, for each material the electrochemical performance for insertion of Li+ ion has been studied by means of various electrochemical techniques to reveal the nature of alkali ion insertion. In addition Na ...
... Li3 Fe2(HPO3)3Cl, LiFe(HPO3)2, Li0.8 Fe(H2O)2B[P2O8]•H2O and AFePO4NO3 (A = NH4/Li, K). Furthermore, for each material the electrochemical performance for insertion of Li+ ion has been studied by means of various electrochemical techniques to reveal the nature of alkali ion insertion. In addition Na ...
Cyclam ``capa` POT.4` to ``capa` POT.3` denticity change
... shows absorptions bands at 264 nm (log ε ) 3.27), 404 nm (log ε ) 2.53), and 532 nm (log ε ) 1.88). 1H and 13C NMR are in agreement with the proposed molecular structure, which shows a very singular architecture where the cyclam ring N (with the carboxypropyl pendant arm) is not coordinated to the r ...
... shows absorptions bands at 264 nm (log ε ) 3.27), 404 nm (log ε ) 2.53), and 532 nm (log ε ) 1.88). 1H and 13C NMR are in agreement with the proposed molecular structure, which shows a very singular architecture where the cyclam ring N (with the carboxypropyl pendant arm) is not coordinated to the r ...
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms).Each contributing structure can be represented by a Lewis structure, with only an integer number of covalent bonds between each pair of atoms within the structure. Several Lewis structures are used collectively to describe the actual molecular structure, which is an approximate intermediate between the canonical forms called a resonance hybrid. Contributing structures differ only in the position of electrons, not in the position of nuclei.Electron delocalization lowers the potential energy of the substance and thus makes it more stable than any of the contributing structures. The difference between the potential energy of the actual structure and that of the contributing structure with the lowest potential energy is called the resonance energy or delocalization energy.Resonance is distinguished from tautomerism and conformational isomerism, which involve the formation of isomers, thus the rearrangement of the nuclear positions.
















![mclintock.ch6 [Compatibility Mode]](http://s1.studyres.com/store/data/003971396_1-780a12aa3165c9221aca3ac594a06674-300x300.png)



