Noncovalent interactions of molecules with single walled carbon
... misalignment may introduce larger differences in binding energy.31 Often, rather than using a discrete potential, a continuum approach has been used,19,32,34–36 where the electronic density is assumed to be uniform over the surface of the nanotube. The continuum and discrete approaches give similar ...
... misalignment may introduce larger differences in binding energy.31 Often, rather than using a discrete potential, a continuum approach has been used,19,32,34–36 where the electronic density is assumed to be uniform over the surface of the nanotube. The continuum and discrete approaches give similar ...
0 CODE COURSE : MP, MF, MR
... 55. Statement-1 : Current is passed through a metallic wire, heating it red. Half of its portion is cooled (by cold water jacket), then rest of the half portion become more hot. ...
... 55. Statement-1 : Current is passed through a metallic wire, heating it red. Half of its portion is cooled (by cold water jacket), then rest of the half portion become more hot. ...
Brief Contents - Educhimica.it
... significant figure in the tenths place after the decimal, and the second number stops its significant figure in the hundredths place after the decimal. Hence, we limit our final answer to the tenths place after the decimal. The final answer is 59.4. b. 0.00665 + 1.004 = 1.01065. The first number stops its ...
... significant figure in the tenths place after the decimal, and the second number stops its significant figure in the hundredths place after the decimal. Hence, we limit our final answer to the tenths place after the decimal. The final answer is 59.4. b. 0.00665 + 1.004 = 1.01065. The first number stops its ...
Chapter 19 Homework Problems Answers
... the cooler object. When in contact with one another, the objects transfer heat through collisions, and eventually, some of the kinetic energy of the molecules in the hot object is transferred to the molecules of the cool object. This process of energy transfer continues until the objects have the sa ...
... the cooler object. When in contact with one another, the objects transfer heat through collisions, and eventually, some of the kinetic energy of the molecules in the hot object is transferred to the molecules of the cool object. This process of energy transfer continues until the objects have the sa ...
Stoichiometry Chapter 3 CHEMA1301 [Compatibility Mode]
... merely memorizing a large number of unrelated reactions. ...
... merely memorizing a large number of unrelated reactions. ...
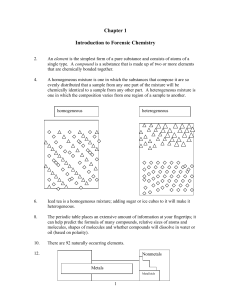
Chapter 1 Introduction to Forensic Chemistry
... Proust did his experimental work very carefully so that he was able to obtain both accurate and precise work. ...
... Proust did his experimental work very carefully so that he was able to obtain both accurate and precise work. ...
Week 3 July 22, 2016 Worksheet Review III 1 mol = 6.022 × 1023 1
... 1.807 × 1024 atoms are present in the sample True. We know that for every 1 molecule of H2O, we have 2 atoms of H and 1 atom of O. So we every 1 molecule of H2O has 3 atoms. 3× (6.022 × 1023 molecules H 2O) = 1.807 × 1024 atoms ...
... 1.807 × 1024 atoms are present in the sample True. We know that for every 1 molecule of H2O, we have 2 atoms of H and 1 atom of O. So we every 1 molecule of H2O has 3 atoms. 3× (6.022 × 1023 molecules H 2O) = 1.807 × 1024 atoms ...
Chapter 9 Review, pages 628–633
... from the carbon atom. Thus the oxidation number of N in HCN(g) is –3. (d) The oxidation number of hydrogen in its compounds is +1, and the oxidation number of oxygen in its compounds is –2. Since there are 2 hydrogen atoms in H2C2O4(aq), the total contribution of the hydrogen atoms is +2. Since ther ...
... from the carbon atom. Thus the oxidation number of N in HCN(g) is –3. (d) The oxidation number of hydrogen in its compounds is +1, and the oxidation number of oxygen in its compounds is –2. Since there are 2 hydrogen atoms in H2C2O4(aq), the total contribution of the hydrogen atoms is +2. Since ther ...
9278654 PS/Chemistry Ja03 - Dolgeville Central School
... 30 Which species can conduct an electric current? (1) NaOH(s) (3) H2O(s) (2) CH3OH(aq) (4) HCl(aq) ...
... 30 Which species can conduct an electric current? (1) NaOH(s) (3) H2O(s) (2) CH3OH(aq) (4) HCl(aq) ...
Chapter 6 Quantities in Chemical Reactions
... atoms, or 1.2044 × 1024 Na atoms. Similarly, if we have 0.5 mol of benzene (C 6H6) molecules, we have 0.5 × (6.022 × 1023) C6H6 molecules, or 3.011 × 1023 C6H6 molecules. ...
... atoms, or 1.2044 × 1024 Na atoms. Similarly, if we have 0.5 mol of benzene (C 6H6) molecules, we have 0.5 × (6.022 × 1023) C6H6 molecules, or 3.011 × 1023 C6H6 molecules. ...
Study Guide for Content Mastery - Student Edition
... a specific situation. There are many kinds of graphs. One of the most common is the bar graph. ...
... a specific situation. There are many kinds of graphs. One of the most common is the bar graph. ...
Section 1.3 - The Student Room
... The formation of a compound from its elements may be an exothermic reaction (DHf negative) or an endothermic reaction (DHf positive). However, energy is liberated whenever a substance burns, so combustion reactions are always exothermic (DHc negative). ...
... The formation of a compound from its elements may be an exothermic reaction (DHf negative) or an endothermic reaction (DHf positive). However, energy is liberated whenever a substance burns, so combustion reactions are always exothermic (DHc negative). ...
Changing Matter
... and 100.0 g of AgNO3 are mixed together? How many grams of the excess reactant remain ...
... and 100.0 g of AgNO3 are mixed together? How many grams of the excess reactant remain ...
Modern Chemistry
... 1. Determine whether each of the following is an example of observation and data, a theory, a hypothesis, a control, or a model. a. A research team records the rainfall in inches per day in a prescribed area of the rain forest. The square footage of vegetation and relative plant density ...
... 1. Determine whether each of the following is an example of observation and data, a theory, a hypothesis, a control, or a model. a. A research team records the rainfall in inches per day in a prescribed area of the rain forest. The square footage of vegetation and relative plant density ...
AQA A-level Chemistry
... reaction the ΔH value for the reverse reaction has the same numerical value as the forward reaction but the sign is changed. This means that for an exothermic reaction in the forward direction ΔH is negative, but in the reverse direction the ΔH value has the same numerical value but it would be posi ...
... reaction the ΔH value for the reverse reaction has the same numerical value as the forward reaction but the sign is changed. This means that for an exothermic reaction in the forward direction ΔH is negative, but in the reverse direction the ΔH value has the same numerical value but it would be posi ...
Preparatory Problems of the 40th IChO - IChO-2016
... –196 °C. The resulting ionic substance (B) was found to comprise three types of atoms. Elemental analysis shows that it contains 9.91 % N and 43.06 % Sb by mass; further, it contains one cation and one anion. The shape of the latter was found to be octahedral. d) ...
... –196 °C. The resulting ionic substance (B) was found to comprise three types of atoms. Elemental analysis shows that it contains 9.91 % N and 43.06 % Sb by mass; further, it contains one cation and one anion. The shape of the latter was found to be octahedral. d) ...
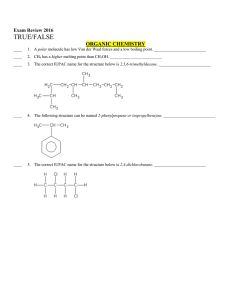
Multiple Choice Exam Review June 2016
... ____ 84. Intermolecular forces are a. forces within covalent molecules that hold them together b. electrostatic forces between ions c. bonds between hydrogen and oxygen atoms in water molecules d. attractive forces between separate covalent molecules e. covalent bonds within a network solid ____ 85 ...
... ____ 84. Intermolecular forces are a. forces within covalent molecules that hold them together b. electrostatic forces between ions c. bonds between hydrogen and oxygen atoms in water molecules d. attractive forces between separate covalent molecules e. covalent bonds within a network solid ____ 85 ...
Descriptive Inorganic Chemistry
... escriptive inorganic chemistry was traditionally concerned with the properties of the elements and their compounds. Now, in the renaissance of the subject, with the synthesis of new and novel materials, the properties are being linked with explanations for the formulas and structures of compounds to ...
... escriptive inorganic chemistry was traditionally concerned with the properties of the elements and their compounds. Now, in the renaissance of the subject, with the synthesis of new and novel materials, the properties are being linked with explanations for the formulas and structures of compounds to ...
Devillez (ld2653) – Test 1 Review – Devillez – (99998)
... deflected, they were deflected at all angles, including some very wide angles! The wide deflections suggested a very hard (dense) positively charged core in the atom. However, this core, or nucleus, must be small in relation to the overall size of the atom, since so few of the α particles were defle ...
... deflected, they were deflected at all angles, including some very wide angles! The wide deflections suggested a very hard (dense) positively charged core in the atom. However, this core, or nucleus, must be small in relation to the overall size of the atom, since so few of the α particles were defle ...
IB Chemistry Online SAQ_Ans
... b Frequency: decreasing; wavelength: increasing, left to right. c When sufficient energy (thermal or electrical) is supplied, electrons can be promoted (excited) to higher energy levels in an atom. The electrons are unstable in higher levels and rapidly emit radiation and fall back into lower ener ...
... b Frequency: decreasing; wavelength: increasing, left to right. c When sufficient energy (thermal or electrical) is supplied, electrons can be promoted (excited) to higher energy levels in an atom. The electrons are unstable in higher levels and rapidly emit radiation and fall back into lower ener ...
AP Chemistry - Siva Kodali
... 2,000 students per year. She is the author of ACT For Dummies, Pre-Calculus For Dummies, AP Biology For Dummies, Chemistry Workbook For Dummies, GRE For Dummies, Pre-Calculus Workbook for Dummies, and other books on self-esteem, writing, and motivational topics. Michelle has overseen dozens of progr ...
... 2,000 students per year. She is the author of ACT For Dummies, Pre-Calculus For Dummies, AP Biology For Dummies, Chemistry Workbook For Dummies, GRE For Dummies, Pre-Calculus Workbook for Dummies, and other books on self-esteem, writing, and motivational topics. Michelle has overseen dozens of progr ...
Massachusetts Tests for Educator Licensure (MTEL )
... Correct Response: D. The combination of chemicals is that of a weak acid and a strong base. This conclusion can be drawn because the equivalence point on the graph corresponds to a pH greater than 7. It is clear that a weak acid is being titrated with a strong base (instead of a strong base being ti ...
... Correct Response: D. The combination of chemicals is that of a weak acid and a strong base. This conclusion can be drawn because the equivalence point on the graph corresponds to a pH greater than 7. It is clear that a weak acid is being titrated with a strong base (instead of a strong base being ti ...
Chemistry - Department of Education and Skills
... considerable discrepancy in the provision of Physics and Chemistry to girls and boys. Since then provision of these subjects has improved, especially in girls’ schools. However, the most recent analysis of provision indicates the persistence of the problem. Although provision for girls is now best i ...
... considerable discrepancy in the provision of Physics and Chemistry to girls and boys. Since then provision of these subjects has improved, especially in girls’ schools. However, the most recent analysis of provision indicates the persistence of the problem. Although provision for girls is now best i ...
ANNEX (Manuscrits posteriors a la Comissió de Doctorat de Juliol del...
... extracted with acidic water and diethyl ether. After chromatography on silica with AcOEt, four different bands were separated. Two of these accounted for more than 90% of the collected masses, and have been the ones studied. These bands correspond to Cs[8,8’-(CH3)2-3,3’-Co(1,2-C2B9H10)2], Cs[3], and ...
... extracted with acidic water and diethyl ether. After chromatography on silica with AcOEt, four different bands were separated. Two of these accounted for more than 90% of the collected masses, and have been the ones studied. These bands correspond to Cs[8,8’-(CH3)2-3,3’-Co(1,2-C2B9H10)2], Cs[3], and ...
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms).Each contributing structure can be represented by a Lewis structure, with only an integer number of covalent bonds between each pair of atoms within the structure. Several Lewis structures are used collectively to describe the actual molecular structure, which is an approximate intermediate between the canonical forms called a resonance hybrid. Contributing structures differ only in the position of electrons, not in the position of nuclei.Electron delocalization lowers the potential energy of the substance and thus makes it more stable than any of the contributing structures. The difference between the potential energy of the actual structure and that of the contributing structure with the lowest potential energy is called the resonance energy or delocalization energy.Resonance is distinguished from tautomerism and conformational isomerism, which involve the formation of isomers, thus the rearrangement of the nuclear positions.




![Stoichiometry Chapter 3 CHEMA1301 [Compatibility Mode]](http://s1.studyres.com/store/data/014247793_1-84b4b6fe6fa37d77afbf7eb657ee347a-300x300.png)